MAIA Biotechnology Files Second Patent For New Telomere-Targeting Molecules Program
Filed provisional new composition of matter patent application for MAIA’s third entirely home-grown telomere-targeting molecule
CHICAGO--(BUSINESS WIRE)-- MAIA Biotechnology, Inc. (NYSE American: MAIA) today announced its second broad provisional patent application covering the composition of matter for a new telomere-targeting molecule. MAIA is creating and evaluating multiple telomere-targeting compounds designed to modify the telomeric structure through the cancer cell - intrinsic telomerase activity - and thus cause the death of these cells. The studies, conducted in vitro in multiple cancer cell lines and in vivo in several pre-clinical cancer models, demonstrated the intended mechanism of action and high-level anti-cancer activity for these new molecules.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20230607005283/en/
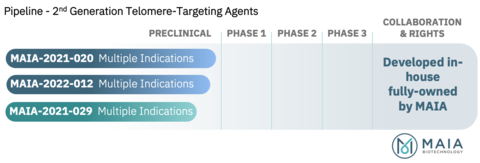
Figure 1. Pipeline of 2nd Generation Telomere-Targeting Agents (Graphic: Business Wire)
MAIA has nominated a new molecular entity candidate (designated as MAIA-2021- 029) for further advancement into preclinical GLP-toxicity and other studies, and may advance this candidate into human clinical trials upon completion of the required preclinical evaluations. The patent titled “TUMOR REDOX-ACTIVATED 6-THIOPURINE CONTAINING DIMER COMPOUNDS” further adds to MAIA’s Telomere-Targeting Molecule Program, which includes THIO, the lead therapeutic candidate currently being evaluated in a Phase 2 clinical trial, and follow-on compounds MAIA-2021-020 and MAIA-2022-012, patented in the fourth quarter of 2022.
“The discovery and preclinical advancements of these new telomere-targeting compounds represent another significant chapter for MAIA. We have observed impressive single-agent activity in several different tumor types for the new candidates, as well as in combination with immune checkpoint inhibitors,” said Sergei Gryaznov, Ph.D., MAIA Chief Scientific Officer. “The observed anti-cancer activity in vitro and in vivo is quite remarkable, often leading to complete tumor eliminations. We are working diligently to advance these candidates toward clinical development.”
“The development of proprietary new molecular entity candidates is a key component to MAIA’s strategy and greatly increases the chances to bring a highly efficacious telomere-targeting therapy to market. Our molecules can be used in the treatment of multiple cancer indications, and with the excellent preliminary results observed in our ongoing Phase II trial evaluating THIO in patients with Non-Small Cell Lung Cancer, we look forward to announcing further developments of MAIA’s proprietary new molecular entity candidates,” said MAIA Chairman and Chief Executive Officer Vlad Vitoc, M.D.
About THIO
THIO (6-thio-dG or 6-thio-2’-deoxyguanosine) is an investigational telomere-targeting agent currently in clinical development to evaluate its activity in Non-Small Cell Lung Cancer (NSCLC). Telomeres, along with the enzyme telomerase, play a fundamental role in the survival of cancer cells and their resistance to current therapies. THIO is being developed as a second or later line of treatment for NSCLC for patients that have progressed beyond the standard-of-care regimen of existing checkpoint inhibitors.
About MAIA Biotechnology, Inc.
MAIA is a targeted therapy, immuno-oncology company focused on the development and commercialization of potential first-in-class drugs with novel mechanisms of action that are intended to meaningfully improve and extend the lives of people with cancer. Our lead program is THIO, a potential first-in-class cancer telomere targeting agent in clinical development for the treatment of NSCLC patients with telomerase-positive cancer cells. For more information, please visit www.maiabiotech.com.
Forward Looking Statements
MAIA cautions that all statements, other than statements of historical facts, contained in this press release, are forward-looking statements. Forward-looking statements are subject to known and unknown risks, uncertainties, and other factors that may cause our or our industry’s actual results, levels or activity, performance or achievements to be materially different from those anticipated by such statements. The use of words such as “may,” “might,” “will,” “should,” “could,” “expect,” “plan,” “anticipate,” “believe,” “estimate,” “project,” “intend,” “future,” “potential,” or “continue,” and other similar expressions are intended to identify forward looking statements. However, the absence of these words does not mean that statements are not forward-looking. For example, all statements we make regarding (i) the initiation, timing, cost, progress and results of our preclinical and clinical studies and our research and development programs, (ii) our ability to advance product candidates into, and successfully complete, clinical studies, (iii) the timing or likelihood of regulatory filings and approvals, (iv) our ability to develop, manufacture and commercialize our product candidates and to improve the manufacturing process, (v) the rate and degree of market acceptance of our product candidates, (vi) the size and growth potential of the markets for our product candidates and our ability to serve those markets, and (vii) our expectations regarding our ability to obtain and maintain intellectual property protection for our product candidates, are forward looking. All forward-looking statements are based on current estimates, assumptions and expectations by our management that, although we believe to be reasonable, are inherently uncertain. Any forward-looking statement expressing an expectation or belief as to future events is expressed in good faith and believed to be reasonable at the time such forward-looking statement is made. However, these statements are not guarantees of future events and are subject to risks and uncertainties and other factors beyond our control that may cause actual results to differ materially from those expressed in any forward-looking statement. Any forward-looking statement speaks only as of the date on which it was made. We undertake no obligation to publicly update or revise any forward-looking statement, whether as a result of new information, future events or otherwise, except as required by law. In this release, unless the context requires otherwise, “MAIA,” “Company,” “we,” “our,” and “us” refers to MAIA Biotechnology, Inc. and its subsidiaries.
View source version on businesswire.com: https://www.businesswire.com/news/home/20230607005283/en/
Investor Inquiries
MAIA Biotechnology
Joseph McGuire
Chief Financial Officer
jmcguire@maiabiotech.com
904-228-2603
Investor Relations
ir@maiabiotech.com
Source: MAIA Biotechnology, Inc.
Released June 7, 2023
