

Telomere Targeting Immunotherapies for Cancer NYSE American: MAIA September 2022 Exhibit 99.1
All statements in this presentation, other than those relating to historical facts, are "forward-looking statements." These forward-looking statements may include, but are not limited to, statements relating to our objectives, plans, and strategies; statements that contain projections of results of operations or of financial condition; statements relating to the industry and government policies and regulations relating to our industry; and all statements (other than statements of historical facts) that address activities, events, or developments that we intend, expect, project, believe, or anticipate will or may occur in the future. Forward-looking statements are not guarantees of future performance and are subject to risks and uncertainties. We have based these forward-looking statements on assumptions and assessments made by our management in light of their experience and their perception of historical trends, current conditions, expected future developments, and other factors they believe to be appropriate. Important factors that could cause actual results, developments, and business decisions to differ materially from those anticipated in these forward-looking statements include, among other things: the overall global economic environment; general market, political, and economic conditions in the countries in which we operate: projected capital expenditures and liquidity; changes in our strategy; government regulations and approvals; the application of certain service license; and litigation and regulatory proceedings. Additional factors that could cause or contribute to differences between the Company's actual results and forward-looking statements include, but are not limited to, those risks discussed in the Company's filings with the U.S. Securities and Exchange Commission (the “SEC”), including, but not limited to, the risks detailed in the Company’s registration statement on Form S-1, as amended (Registration No.: 333-264225), and any subsequent filings with the SEC. You may get these documents for free by visiting EDGAR on the Commission's website at www.sec.gov. We caution you that forward-looking statements are not guarantees of future performance and that our actual results of operations, financial condition and liquidity, and the development of the industry in which we operate may differ materially from the forward looking statements contained in this presentation as a result of, among other factors, the factors referenced in the "Risk Factors” section of the Registration Statement. In addition, even if our results of operations, financial condition and liquidity, and the development of the industry in which we operate are consistent with the forward-looking statements contained in this presentation, they may not be predictive of results or developments in future periods. These statements are only current predictions and are subject to known and unknown risks, uncertainties, and other factors that may cause our or our industry's actual results, levels of activity, performance, or achievements to be materially different from those anticipated by the forward-looking statements. You should not rely upon forward-looking statements as predictions of future events. Although we believe that the expectations reflected in the forward-looking statements are reasonable, we cannot guarantee future results, levels of activity, performance, or achievements. Except as required by law, we are under no duty to update or revise any of the forward-looking statements, whether as a result of new information, future events or otherwise, after the date of this prospectus. These forward-looking statements speak only as of the date of this presentation, and we assume no obligation to update or revise these forward-looking statements for any reason. Disclosure
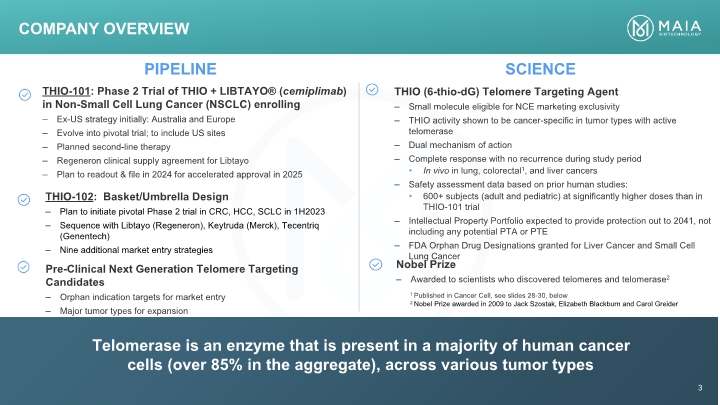
PIPELINE SCIENCE Telomerase is an enzyme that is present in a majority of human cancer cells (over 85% in the aggregate), across various tumor types Company Overview 3 1 Published in Cancer Cell, see slides 28-30, below 2 Nobel Prize awarded in 2009 to Jack Szostak, Elizabeth Blackburn and Carol Greider THIO-101: Phase 2 Trial of THIO + LIBTAYO® (cemiplimab) in Non-Small Cell Lung Cancer (NSCLC) enrolling – Ex-US strategy initially: Australia and Europe – Evolve into pivotal trial; to include US sites – Planned second-line therapy – Regeneron clinical supply agreement for Libtayo – Plan to readout & file in 2024 for accelerated approval in 2025 THIO-102: Basket/Umbrella Design – Plan to initiate pivotal Phase 2 trial in CRC, HCC, SCLC in 1H2023 – Sequence with Libtayo (Regeneron), Keytruda (Merck), Tecentriq (Genentech) – Nine additional market entry strategies Pre-Clinical Next Generation Telomere Targeting Candidates – Orphan indication targets for market entry – Major tumor types for expansion THIO (6-thio-dG) Telomere Targeting Agent – Small molecule eligible for NCE marketing exclusivity – THIO activity shown to be cancer-specific in tumor types with active telomerase – Dual mechanism of action – Complete response with no recurrence during study period • In vivo in lung, colorectal1 , and liver cancers – Safety assessment data based on prior human studies: • 600+ subjects (adult and pediatric) at significantly higher doses than in THIO-101 trial – Intellectual Property Portfolio expected to provide protection out to 2041, not including any potential PTA or PTE – FDA Orphan Drug Designations granted for Liver Cancer and Small Cell Lung Cancer Nobel Prize – Awarded to scientists who discovered telomeres and telomerase 2
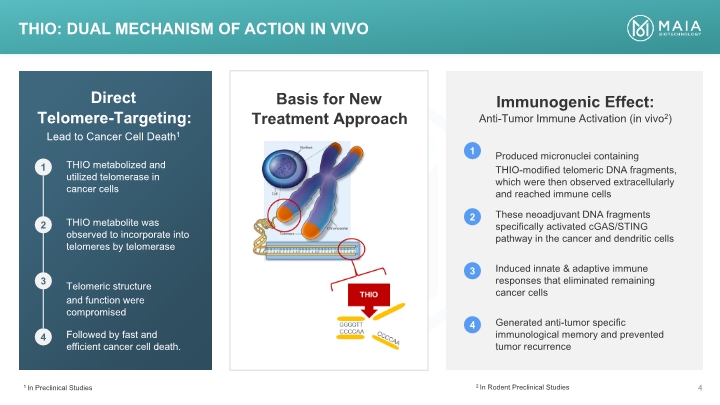
Basis for New Treatment Approach Direct Telomere-Targeting: Lead to Cancer Cell Death1 Immunogenic Effect: Anti-Tumor Immune Activation (in vivo2) THIO: Dual mechanism of action In VIVO 1 In Preclinical Studies 2 In Rodent Preclinical Studies Lead to Cancer Cell Death1 1 THIO metabolized and utilized telomerase in cancer cells 2 THIO metabolite was observed to incorporate into telomeres by telomerase 3 Telomeric structure and function were compromised 4 Followed by fast and efficient cancer cell death. 1 Produced micronuclei containing THIO-modified telomeric DNA fragments, which were then observed extracellularly and reached immune cells 2 These neoadjuvant DNA fragments specifically activated cGAS/STING pathway in the cancer and dendritic cells 3 Induced innate & adaptive immune responses that eliminated remaining cancer cells 4 Generated anti-tumor specific immunological memory and prevented tumor recurrence
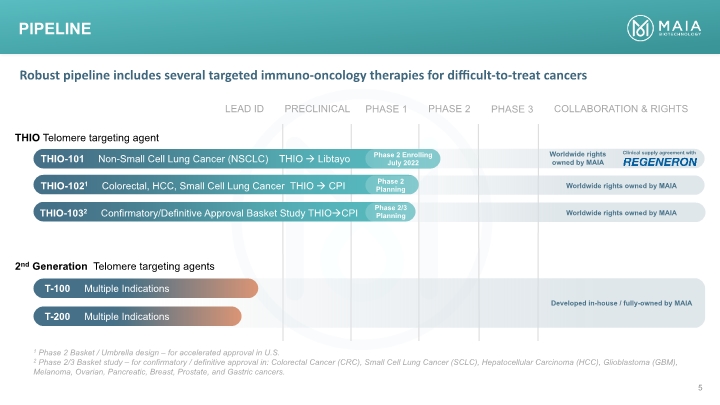
Pipeline THIO Telomere targeting agent THIO-101 Non-Small Cell Lung Cancer (NSCLC) THIO Libtayo 2nd Generation Telomere targeting agents Phase 2 Enrolling July 2022 THIO-1021 Colorectal, HCC, Small Cell Lung Cancer THIO CPI THIO-1032 Confirmatory/Definitive Approval Basket Study THIOCPI Phase 2 Planning Phase 2/3 Planning T-100 Multiple Indications T-200 Multiple Indications Worldwide rights owned by MAIA Worldwide rights owned by MAIA Worldwide rights owned by MAIA Clinical supply agreement with Developed in-house / fully-owned by MAIA 1 Phase 2 Basket / Umbrella design – for accelerated approval in U.S. 2 Phase 2/3 Basket study – for confirmatory / definitive approval in: Colorectal Cancer (CRC), Small Cell Lung Cancer (SCLC), Hepatocellular Carcinoma (HCC), Glioblastoma (GBM), Melanoma, Ovarian, Pancreatic, Breast, Prostate, and Gastric cancers. Robust pipeline includes several targeted immuno-oncology therapies for difficult-to-treat cancers PRECLINICAL PHASE 1 PHASE 2 PHASE 3 LEAD ID COLLABORATION & RIGHTS

Goal of Study: New Standard of Care for Treatment of NSCLC Phase 2 dose optimization study, to evolve into a pivotal study for US approval: Safety lead-in Select optimal dose for planned pivotal study Contribute Drug Supply LIBTAYO®️ (cemiplimab; anti-PD1) Development exclusivity only for NSCLC for study period All other tumor types remain open Regeneron clinical supply agreement - Libtayo Regeneron clinical supply agreement – Libtayo Goal of Study: New Standard of Care for Treatment of NSCLC Phase 2 dose optimization study, to evolve into a pivotal study for US approval: Safety lead-in Select optimal dose for planned pivotal study Contribute Drug Supply LIBTAYO®️ (cemiplimab; anti-PD1) Development exclusivity only for NSCLC for study period All other tumor types remain open Established Joint Development Collaboration Committee to maximize success Maia biotechnology inc Announces clincal supply agreement with regeneron for phase ½ clinical trial evaluating thio in sequential administration with libitayo (cemiplimb) in advanced non small cell lung cancer February 02,2021 08:00 am eastern standard time Chicago {business wire} maia biotechnology inc a target therapy immune oncology company focused on development of firest in class oncology drugs today announced a clinical supply agreement with regeneron pharmaceuticals, inc (REGN) to evaluate THIO (aka 6 thio dg followed by the pd-1 inhibitor libtayo (cemiplimab) in a phase ½ clinical trial in second-line or later advanced non small cell lung cancer(NSCLC) patients who have progressed following treatment with standard of care regimen that include a checkpoint inhibitor. This clinical trial will evaluate the safety and efficacy of four dose the lead in portion of the study wil initially assess the safety and immunogenic effects of each of the thio doses and overallresponse rate (ORR) as the basis for potentially expanding individual patient cohorts and evaluation in other cancer types. The phase ½ clinical trial is expected to beign enrolling patientsin 2021 "We are excited for the opportunity to partner with Regeneron on our 'Priming tumors with THIO before planned clinical trial of THIO and believe this collaboration to be validating Libtayo treatment is a novel approachof the program's potential to transform both the immuno-oncology that may enhance and extend thelandscape and the cancer treatment paradigm," stated Vlad Vitoc, MD, potential benefits of immunotherapy forMAIA's Chief Executive Officer and President. "Notably, THIO has a welldemonstrated clinical safety profile at varying dosage levels and in patients with advanced non-small cell preclinical results, low-dose THIO followed by immunotherapy has shown lung cancer, and we look forward tocomplete elimination of advanced tumors with no indication ot treatment seeing if the positive pre-clinical resultslimiting toxicity. The efficacy results of this trial are expected to support the that MAIA has published will translate tocontinued development of THIO in NSCLC and potentially its expansion to treat a the clinic."vast array of other cancers. Based on our extensive preclinical experience, we believe that THIO may transform immunologically 'cold' Tweet thistumors into 'hot', rendering them responsive to standard-of-care immunooncology therapies, and potentially improving their effectiveness."
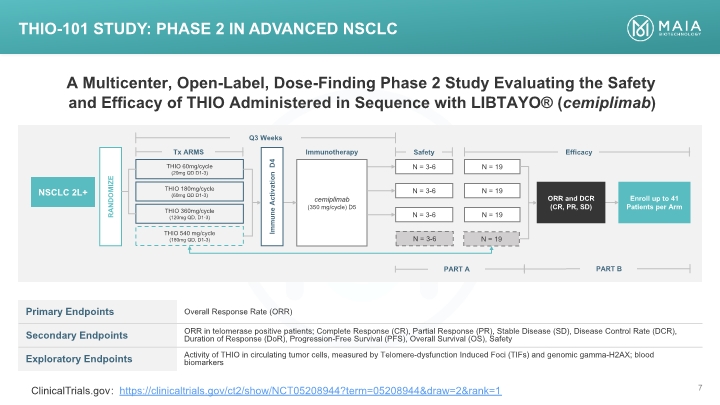
A Multicenter, Open-Label, Dose-Finding Phase 2 Study Evaluating the Safety and Efficacy of THIO Administered in Sequence with LIBTAYO® (cemiplimab) THIO-101 Study: Phase 2 in Advanced NSCLC ClinicalTrials.gov: https://clinicaltrials.gov/ct2/show/NCT05208944?term=05208944&draw=2&rank=1 THIO-101 Study: Phase 2 in Advanced NSCLC A Multicenter, Open-Label, Dose-Finding Phase 2 Study Evaluating the Safety and Efficacy of THIO Administered in Sequence with LIBTAYO® (cemiplimab) [BAR CHART] Primary Endpoints Overall Response Rate (ORR) Secondary Endpoints ORR in telomerase positive patients; Complete Response (CR), Partial Response (PR), Stable Disease (SD), Disease Control Rate (DCR), Duration of Response (DoR), Progression-Free Survival (PFS), Overall Survival (OS), Safety Exploratory Endpoints Activity of THIO in circulating tumor cells, measured by Telomere-dysfunction Induced Foci (TIFs) and genomic gamma-H2AX; blood biomarkers ClinicalTrials.gov: https://clinicaltrials.gov/ct2/show/NCT05208944?term=05208944&draw=2&rank=1 MAIA BIOTECHNOLOGY 7
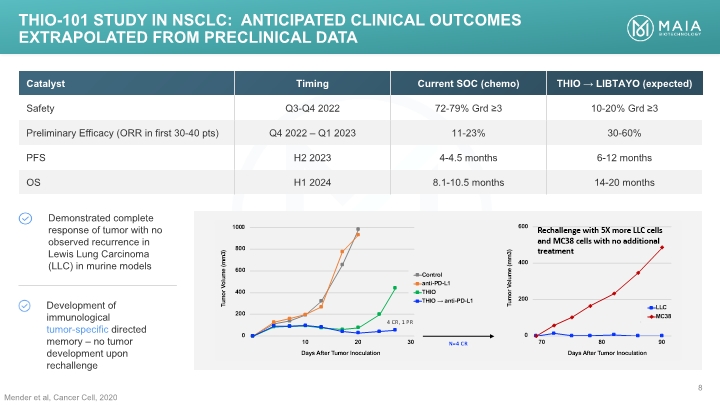
Mender et al, Cancer Cell, 2020 THIO-101 Study IN NSCLC: anticipated CLINICAL OUTCOMES EXTRAPOLATED FROM PRECLINICAL DATA THIO-101 Study IN NSCLC: anticipated CLINICAL OUTCOMES EXTRAPOLATED FROM PRECLINICAL DATA MAIA BIOTECHNOLOGY 8 Catalyst Timing Current SOC (chemo) THIO LIBTAYO (expected) Safety Q3-Q4 2022 72-79% Grd ≥3 10-20% Grd ≥3 Preliminary Efficacy (ORR in first 30-40 pts) Q4 2022 – Q1 2023 11-23% 30-60% PFS H2 2023 4-4.5 months 6-12 months OS H1 2024 8.1-10.5 months 14-20 months Demonstrated complete response of tumor with no observed recurrence in Lewis Lung Carcinoma (LLC) in murine models Development of immunological tumor-specific directed memory – no tumor development upon rechallenge [BAR CHART]
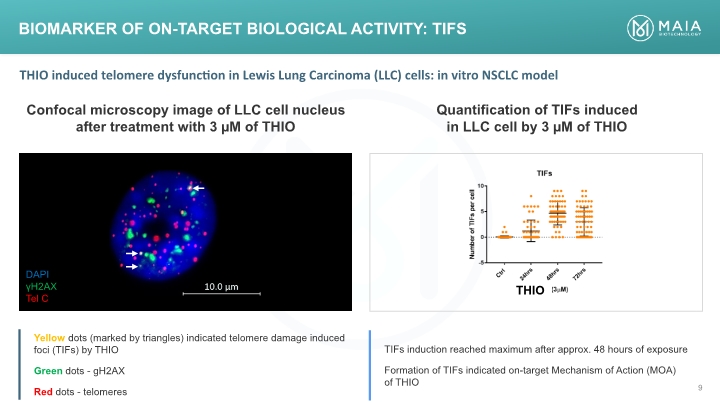
BIOMARKER OF ON-TARGET BIOLOGICAL ACTIVITY: TIFS DAPI γH2AX Tel C Confocal microscopy image of LLC cell nucleus after treatment with 3 µM of THIO Quantification of TIFs induced in LLC cell by 3 µM of THIO THIO THIO induced telomere dysfunction in Lewis Lung Carcinoma (LLC) cells: in vitro NSCLC model Yellow dots (marked by triangles) indicated telomere damage induced foci (TIFs) by THIO Green dots - gH2AX Red dots – telomeres TIFs induction reached maximum after approx. 48 hours of exposure Formation of TIFs indicated on-target Mechanism of Action (MOA) of THIO DAPI γH2AX Tel C (Bar Chart)
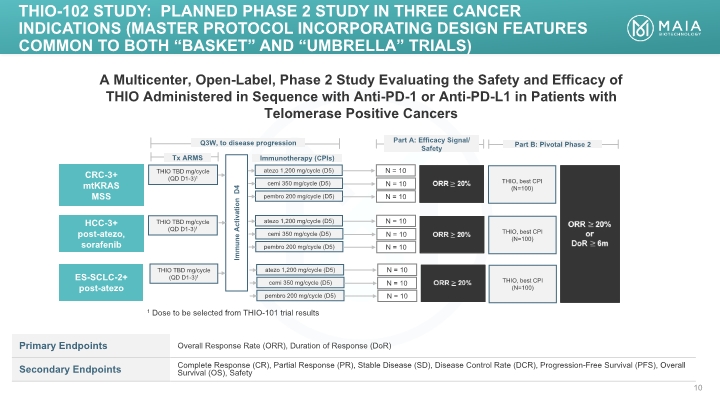
A Multicenter, Open-Label, Phase 2 Study Evaluating the Safety and Efficacy of THIO Administered in Sequence with Anti-PD-1 or Anti-PD-L1 in Patients with Telomerase Positive Cancers THIO TBD mg/cycle (QD D1-3)1 ORR 20% N = 10 Tx ARMS CRC-3+ mtKRAS MSS Immunotherapy (CPIs) Immune Activation D4 Q3W, to disease progression THIO-102 Study: PLANNED Phase 2 study IN THREE CANCER INDICATIONS (MASTER PROTOCOL INCORPORATING DESIGN FEATURES COMMON TO BOTH “basket” and “UMBRELLA” Trials) atezo 1,200 mg/cycle (D5) ORR 20% or DoR 6m Part B: Pivotal Phase 2 THIO TBD mg/cycle (QD D1-3)1 THIO TBD mg/cycle (QD D1-3)1 HCC-3+ post-atezo, sorafenib ES-SCLC-2+ post-atezo cemi 350 mg/cycle (D5) pembro 200 mg/cycle (D5) N = 10 N = 10 ORR 20% N = 10 atezo 1,200 mg/cycle (D5) cemi 350 mg/cycle (D5) pembro 200 mg/cycle (D5) N = 10 N = 10 ORR 20% N = 10 atezo 1,200 mg/cycle (D5) cemi 350 mg/cycle (D5) pembro 200 mg/cycle (D5) N = 10 N = 10 1 Dose to be selected from THIO-101 trial results THIO, best CPI (N=100) THIO, best CPI (N=100) THIO, best CPI (N=100)
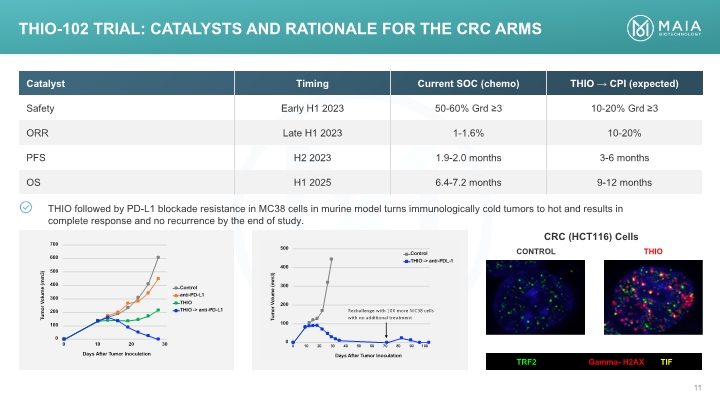
TRF2 Gamma- H2AX TIF CRC (HCT116) Cells THIO-102 TRIAL: Catalysts AND RATIONALE for the CRC arms
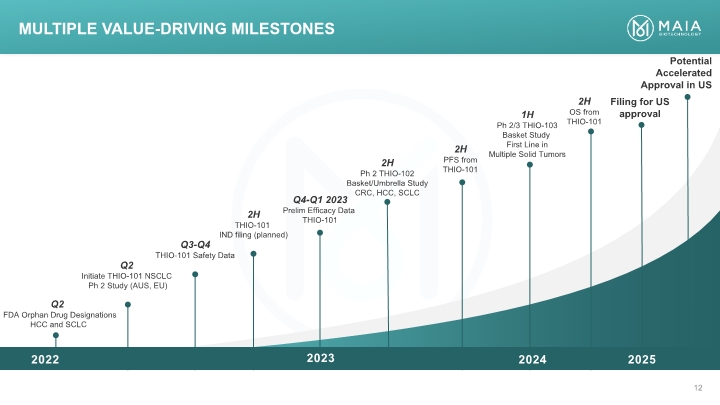
Q2 FDA Orphan Drug Designations HCC and SCLC Q3-Q4 THIO-101 Safety Data Q4-Q1 2023 Prelim Efficacy Data THIO-101 1H Ph 2/3 THIO-103 Basket Study First Line in Multiple Solid Tumors Potential Accelerated Approval in US 2H Ph 2 THIO-102 Basket/Umbrella Study CRC, HCC, SCLC 2H THIO-101 IND filing (planned) Q2 Initiate THIO-101 NSCLC Ph 2 Study (AUS, EU) 2H OS from THIO-101 2H PFS from THIO-101 Multiple Value-Driving Milestones Filing for US approval 2024
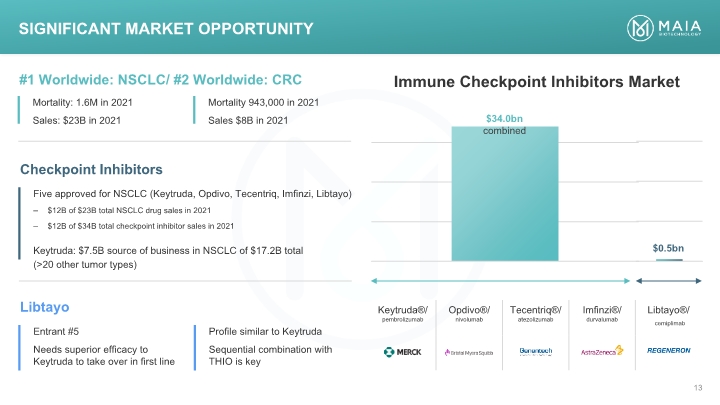
Immune Checkpoint Inhibitors Market Significant Market Opportunity Significant Market Opportunity #1 Worldwide: NSCLC/ #2 Worldwide: CRC Mortality: 1.6M in 2021 Sales: $23B in 2021 Mortality 943,000 in 2021 Sales $8B in 2021 Immune Checkpoint Inhibitors Market Checkpoint Inhibitors $34.0bn combined $0.5bn Five approved for NSCLC (Keytruda, Opdivo, Tecentriq, Imfinzi, Libtayo) $12B of $23B total NSCLC drug sales in 2021 $12B of $34B total checkpoint inhibitor sales in 2021 Keytruda: $7.5B source of business in NSCLC of $17.2B total (>20 other tumor types) Libtayo Entrant #5 Needs superior efficacy to Keytruda to take over in first line Profile similar to Keytruda Sequential combination with THIO is key Keytruda®/ pembrolizumab merck Opdivo®/ nivolumab Bristol myers squibb Tecentriq®/ atezolizumab Genetech A member of the roche group Imfinzi®/ durvalumab Astrazeneca Libtayo®/ cemiplimab Regeneron
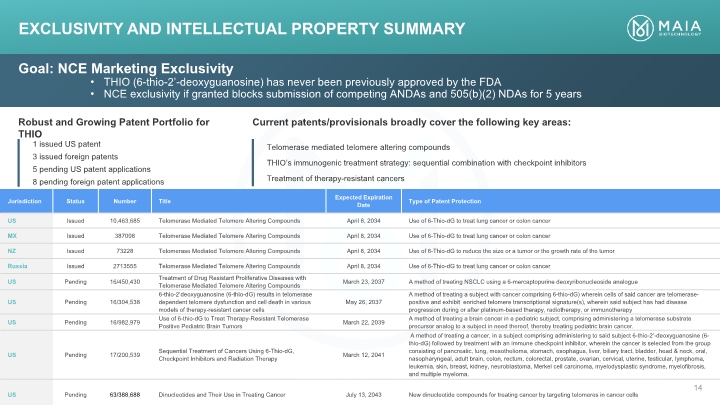
Goal: NCE Marketing Exclusivity THIO (6-thio-2’-deoxyguanosine) has never been previously approved by the FDA NCE exclusivity if granted blocks submission of competing ANDAs and 505(b)(2) NDAs for 5 years Robust and Growing Patent Portfolio for THIO 1 issued US patent 3 issued foreign patents 5 pending US patent applications 8 pending foreign patent applications Current patents/provisionals broadly cover the following key areas: Telomerase mediated telomere altering compounds THIO’s immunogenic treatment strategy: sequential combination with checkpoint inhibitors Treatment of therapy-resistant cancers EXCLUSIVITY AND Intellectual Property Summary EXCLUSIVITY AND Intellectual Property Summary Goal: NCE Marketing Exclusivity THIO (6-thio-2’-deoxyguanosine) has never been previously approved by the FDA NCE exclusivity if granted blocks submission of competing ANDAs and 505(b)(2) NDAs for 5 years Robust and Growing Patent Portfolio for THIO Current patents/provisionals broadly cover the following key areas: 1 issued US patent 3 issued foreign patents 5 pending US patent applications 8 pending foreign patent applications Telomerase mediated telomere altering compounds THIO’s immunogenic treatment strategy: sequential combination with checkpoint inhibitors Treatment of therapy-resistant cancers Jurisdiction Status Number Title Expected Expiration Date Type of Patent Protection US Issued 10,463,685 Telomerase Mediated Telomere Altering Compounds April 8, 2034 Use of 6-Thio-dG to treat lung cancer or colon cancer MX Issued 387008 Telomerase Mediated Telomere Altering Compounds April 8, 2034 Use of 6-Thio-dG to treat lung cancer or colon cancer NZ Issued 73228 Telomerase Mediated Telomere Altering Compounds April 8, 2034 Use of 6-Thio-dG to reduce the size or a tumor or the growth rate of the tumor Russia Issued 2713555 Telomerase Mediated Telomere Altering Compounds April 8, 2034 Use of 6-Thio-dG to treat lung cancer or colon cancer US Pending 16/450,430 Treatment of Drug Resistant Proliferative Diseases with Telomerase Mediated Telomere Altering Compounds March 23, 2037 A method of treating NSCLC using a 6-mercaptopurine deoxyribonucleoside analogue US Pending 16/304,538 6-thio-2’deoxyguanosine (6-thio-dG) results in telomerase dependent telomere dysfunction and cell death in various models of therapy-resistant cancer cells May 26, 2037 A method of treating a subject with cancer comprising 6-thio-dG) wherein cells of said cancer are telomerase-positive and exhibit enriched telomere transcriptional signature(s), wherein said subject has had disease progression during or after platinum-based therapy, radiotherapy, or immunotherapy US Pending 16/982,979 Use of 6-thio-dG to Treat Therapy-Resistant Telomerase Positive Pediatric Brain Tumors March 22, 2039 A method of treating a brain cancer in a pediatric subject, comprising administering a telomerase substrate precursor analog to a subject in need thereof, thereby treating pediatric brain cancer. US Pending 17/200,539 Sequential Treatment of Cancers Using 6-Thio-dG, Checkpoint Inhibitors and Radiation Therapy March 12, 2041 A method of treating a cancer, in a subject comprising administering to said subject 6-thio-2’-deoxyguanosine (6-thio-dG) followed by treatment with an immune checkpoint inhibitor, wherein the cancer is selected from the group consisting of pancreatic, lung, mesothelioma, stomach, esophagus, liver, biliary tract, bladder, head & neck, oral, nasopharyngeal, adult brain, colon, rectum, colorectal, prostate, ovarian, cervical, uterine, testicular, lymphoma, leukemia, skin, breast, kidney, neuroblastoma, Merkel cell carcinoma, myelodysplastic syndrome, myelofibrosis, and multiple myeloma. US Pending 63/388,688 Dinucleotides and Their Use in Treating Cancer July 13, 2043 New dinucleotide compounds for treating cancer by targeting telomeres in cancer cells
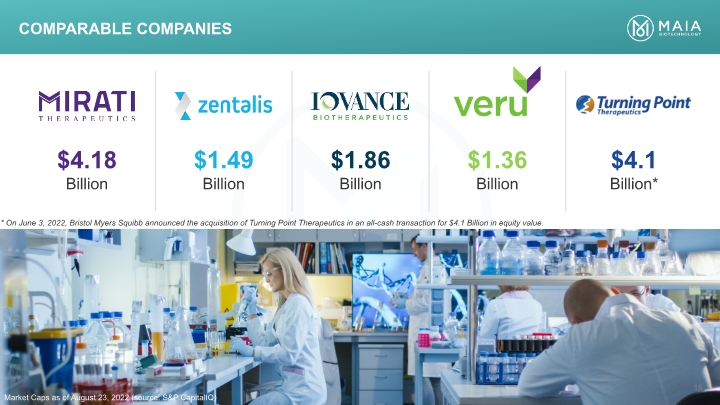
Comparable companies Market Caps as of August 23, 2022 (source: S&P CapitalIQ) 15 * On June 3, 2022, Bristol Myers Squibb announced the acquisition of Turning Point Therapeutics in an all-cash transaction for $4.1 Billion in equity value.
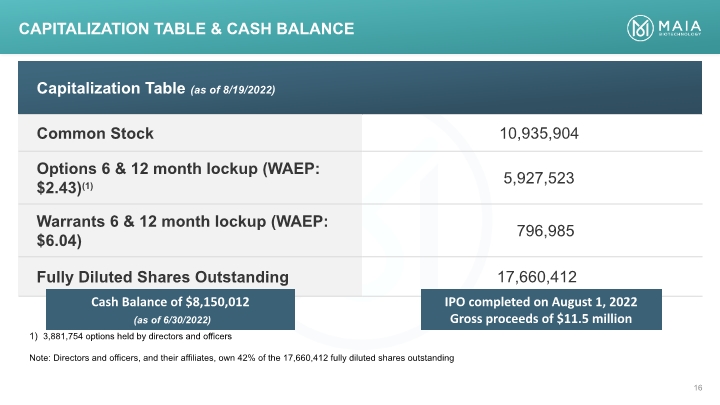
Capitalization Table & CASH BALANCE 3,881,754 options held by directors and officers Note: Directors and officers, and their affiliates, own 42% of the 17,660,412 fully diluted shares outstanding Cash Balance of $8,150,012 (as of 6/30/2022) IPO completed on August 1, 2022 Gross proceeds of $11.5 million

22+ yrs. Pharma/Biotech: Commercial, Medical, 12 compounds launched, 20+ tumor types, 8 Oncology companies. Served in Oncology leadership roles at Cephalon (Treanda), Astellas (Tarceva, Xtandi), Bayer (Nexavar), Novartis (Zometa), and Incyte (Jakafi) 25+ yrs. Scientist/Expert Drug Discovery and Development, Oncology, 120+ publications and Head of the J&J Oligonucleotide Center of Excellence Worldwide recognized expert of telomeres and telomerase in cancer Co-inventor of THIO Hematologist/Oncologist executive with over 21 years of drug development experience: cell therapy, active immunotherapy and cancer vaccines, antibodies, antibody drug conjugates (ADCs), small molecules Vlad Vitoc, MD, MBA Founder, Chairman, and Chief Executive Officer Sergei Gryaznov, PhD Chief Scientific Officer Mihail Obrocea, MD Chief Medical Officer 30+ yrs. Serving as CFO for privately held and publicly traded companies in the health care, financial services, investment, and manufacturing industries. Joe McGuire Chief Financial Officer President, U.S. Markets & Consumer Interactive, overseeing two TransUnion business lines. U.S. Markets provides information and insights to business customers across financial services, insurance, public sector, media and diversified markets. Steven M. Chaouki Board Member 30 years of service with HSBC Group in Global Banking and Markets including investment and securities management, asset management, and global research. Held key leadership positions within Group Internal Audit of HSBC in Latin America, Asia Pacific, and United Kingdom. Adelina Louie Ngar Yee Board Member Highly Experienced Management Team

Investment Highlights 18 Investment Highlights Two Clinical Programs – Ph 2 THIO-101 in NSCLC: THIO + Libtayo® (cemiplimab) Regeneron; Enrolling – Ph 2 THIO-102 in CRC, HCC, and SCLC: THIO + CPIs; Planned 2023 THIO is a Unique Direct Telomere Targeting Agent – Potential to be used in combination with other anticancer and immune therapies – Dual, novel mechanism of action – Plan to make existing drugs better Partnership with Major Pharma – Regeneron: relationship started at early stage of THIO development – Potentially expand existing relationship and target new companies Strong and Growing IP Portfolio – Potential for receiving NCE marketing exclusivity – 4 patents issued, 13 patent applications pending Next Generation Potential Telomere Targeting Therapeutics – Develop two pre-clinical products in new indications – Expand beyond immune checkpoint inhibitor combinations – Seasoned Management Team 1 2 3 4 5 6 MAIA BIOTECHNOLOGY

Telomere Targeting Immunotherapies for Cancer August 2022
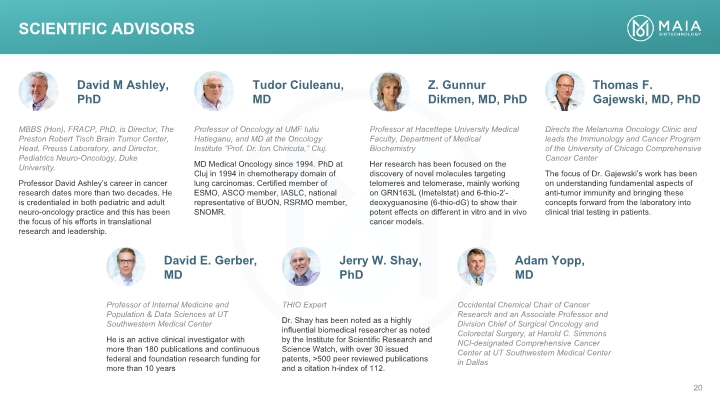
MBBS (Hon), FRACP, PhD, is Director, The Preston Robert Tisch Brain Tumor Center, Head, Preuss Laboratory, and Director, Pediatrics Neuro-Oncology, Duke University. Professor David Ashley’s career in cancer research dates more than two decades. He is credentialed in both pediatric and adult neuro-oncology practice and this has been the focus of his efforts in translational research and leadership. David M Ashley, PhD Professor of Oncology at UMF Iuliu Hatieganu, and MD at the Oncology Institute “Prof. Dr. Ion Chiricuta,” Cluj. MD Medical Oncology since 1994. PhD at Cluj in 1994 in chemotherapy domain of lung carcinomas. Certified member of ESMO, ASCO member, IASLC, national representative of BUON, RSRMO member, SNOMR. Professor at Hacettepe University Medical Faculty, Department of Medical Biochemistry Her research has been focused on the discovery of novel molecules targeting telomeres and telomerase, mainly working on GRN163L (Imetelstat) and 6-thio-2’-deoxyguanosine (6-thio-dG) to show their potent effects on different in vitro and in vivo cancer models. Directs the Melanoma Oncology Clinic and leads the Immunology and Cancer Program of the University of Chicago Comprehensive Cancer Center The focus of Dr. Gajewski’s work has been on understanding fundamental aspects of anti-tumor immunity and bringing these concepts forward from the laboratory into clinical trial testing in patients. Tudor Ciuleanu, MD Z. Gunnur Dikmen, MD, PhD Thomas F. Gajewski, MD, PhD Professor of Internal Medicine and Population & Data Sciences at UT Southwestern Medical Center He is an active clinical investigator with more than 180 publications and continuous federal and foundation research funding for more than 10 years David E. Gerber, MD THIO Expert Dr. Shay has been noted as a highly influential biomedical researcher as noted by the Institute for Scientific Research and Science Watch, with over 30 issued patents, >500 peer reviewed publications and a citation h-index of 112. Jerry W. Shay, PhD Occidental Chemical Chair of Cancer Research and an Associate Professor and Division Chief of Surgical Oncology and Colorectal Surgery, at Harold C. Simmons NCI-designated Comprehensive Cancer Center at UT Southwestern Medical Center in Dallas Adam Yopp, MD Scientific Advisors
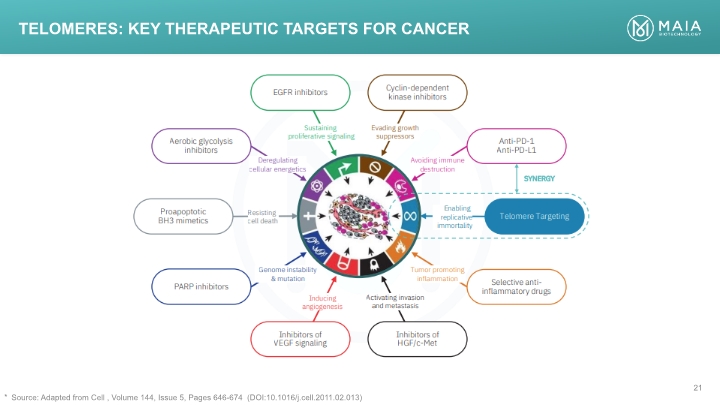
Telomeres: Key Therapeutic Targets for Cancer Source: Adapted from Cell , Volume 144, Issue 5, Pages 646-674 (DOI:10.1016/j.cell.2011.02.013) EGFR inhibitors Cyclin-dependent kinase inhibitors Inhibitors of HGF/c-Met Inhibitors of VEGF signaling Selective anti-inflammatory drugs PARP inhibitors Telomere Targeting Anti-PD-1 Anti-PD-L1 Proapoptotic BH3 mimetics Aerobic glycolysis inhibitors Deregulating cellular energetics Sustaining proliferative signaling Evading growth suppressors Avoiding immune destruction Resisting cell death Inducing angiogenesis Activating invasion and metastasis Tumor promoting inflammation Enabling replicative immortality Genome instability & mutation SYNERGY
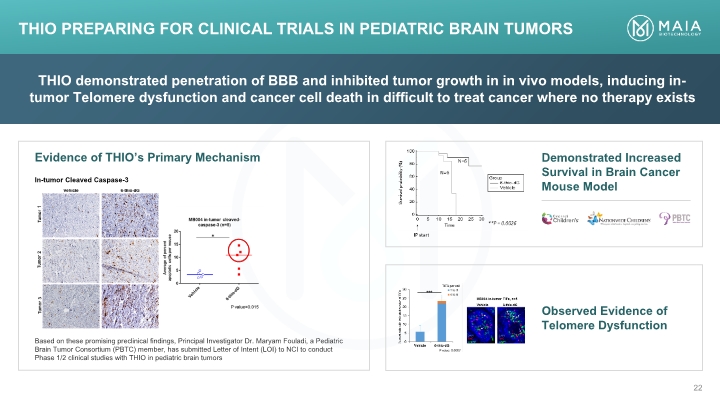
THIO demonstrated penetration of BBB and inhibited tumor growth in in vivo models, inducing in-tumor Telomere dysfunction and cancer cell death in difficult to treat cancer where no therapy exists Demonstrated Increased Survival in Brain Cancer Mouse Model Observed Evidence of Telomere Dysfunction THIO PREPARING for Clinical Trials in Pediatric Brain Tumors
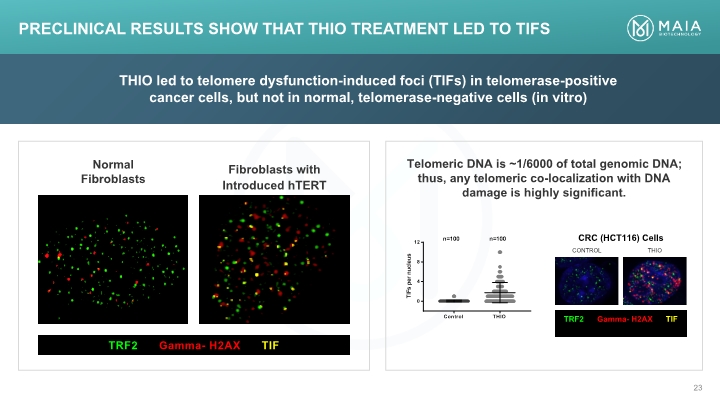
THIO led to telomere dysfunction-induced foci (TIFs) in telomerase-positive cancer cells, but not in normal, telomerase-negative cells (in vitro) Telomeric DNA is ~1/6000 of total genomic DNA; thus, any telomeric co-localization with DNA damage is highly significant. Preclinical Results Show that THIO Treatment Led to TIFs
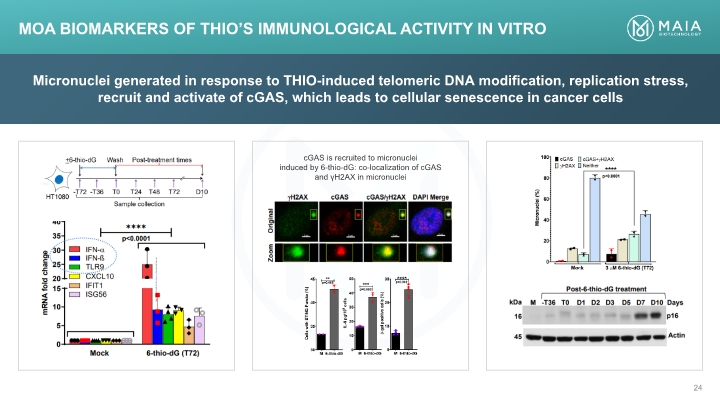
Micronuclei generated in response to THIO-induced telomeric DNA modification, replication stress, recruit and activate of cGAS, which leads to cellular senescence in cancer cells MoA Biomarkers of THIO’s Immunological Activity In Vitro
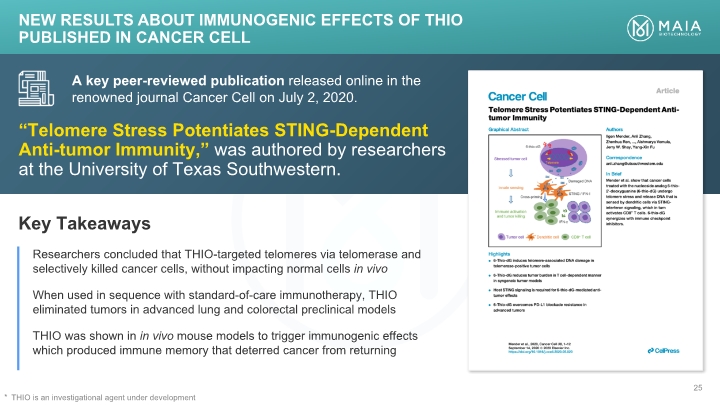
“Telomere Stress Potentiates STING-Dependent Anti-tumor Immunity,” was authored by researchers at the University of Texas Southwestern. A key peer-reviewed publication released online in the renowned journal Cancer Cell on July 2, 2020. Key Takeaways Researchers concluded that THIO-targeted telomeres via telomerase and selectively killed cancer cells, without impacting normal cells in vivo When used in sequence with standard-of-care immunotherapy, THIO eliminated tumors in advanced lung and colorectal preclinical models THIO was shown in in vivo mouse models to trigger immunogenic effects which produced immune memory that deterred cancer from returning NEW Results about Immunogenic Effects of THIO Published in Cancer Cell THIO is an investigational agent under development
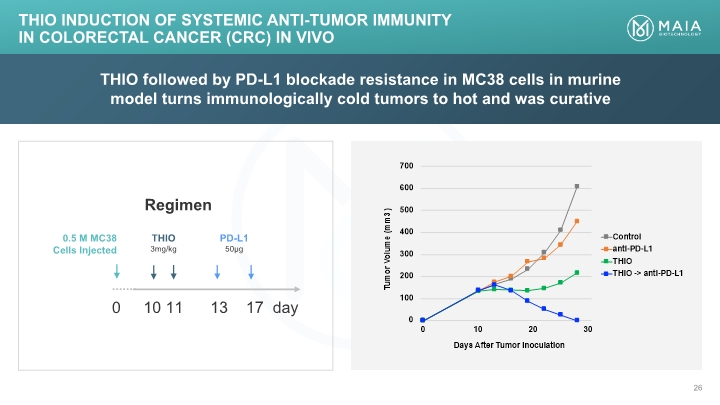
THIO followed by PD-L1 blockade resistance in MC38 cells in murine model turns immunologically cold tumors to hot and was curative THIO Induction of Systemic Anti-tumor Immunity in Colorectal Cancer (CRC) In Vivo
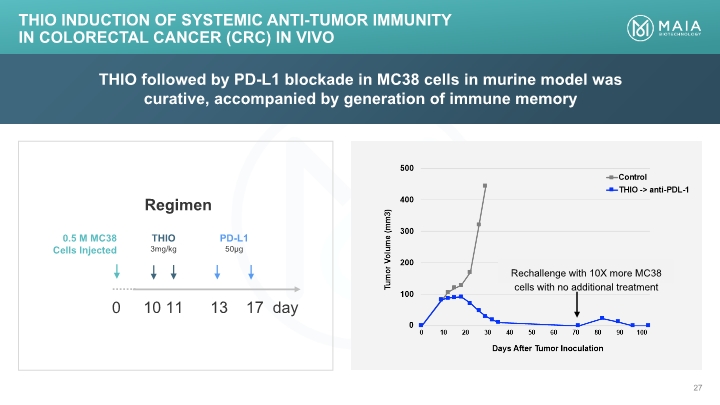
THIO followed by PD-L1 blockade in MC38 cells in murine model was curative, accompanied by generation of immune memory THIO Induction of Systemic Anti-tumor Immunity in Colorectal Cancer (CRC) In Vivo
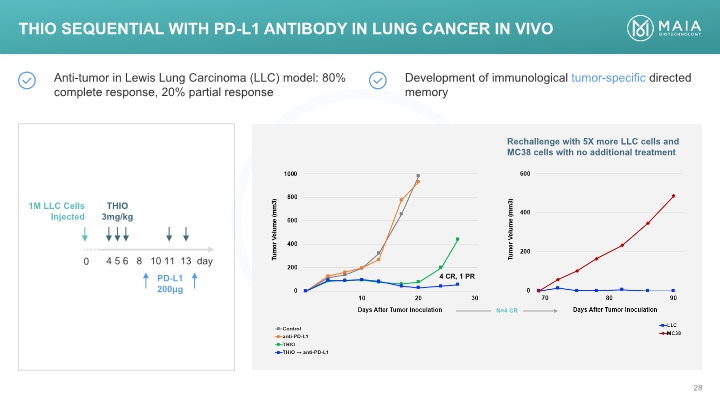
THIO Sequential with PD-L1 Antibody in Lung Cancer In Vivo THIO Sequential with PD-L1 Antibody in Lung Cancer In Vivo Anti-tumor in Lewis Lung Carcinoma (LLC) model: 80% complete response, 20% partial response Development of immunological tumor-specific directed memory 1M LLC Cells Injected THIO 3mg/kg 4 5 6 8 10 11 13 day PD-L1 200μg Rechallenge with 5X more LLC cells and MC38 cells with no additional treatment 1000 800 600 400 200 0 10 20 30 4 CR, PR N=4 CR Days After Tumor Inoculation Tumor Volume (mm3) Control Anti-PD-L1 THIO THIO Anti-PD-L1 600 400 200 0 70 80 90 Tumor Volume (mm3) Days After Tumor Inoculation LLC MC-38 MAIA BIOTECHNOLOGY
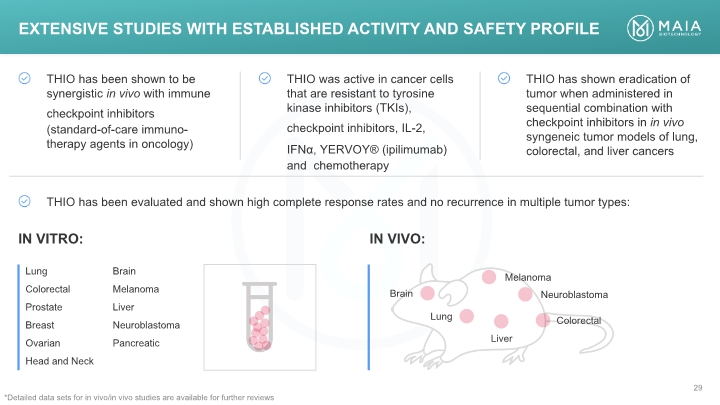
IN VIVO: IN VITRO: Extensive Studies with Established Activity and Safety Profile *Detailed data sets for in vivo/in vivo studies are available for further reviews Extensive Studies with Established Activity and Safety Profile \THIO has been shown to be synergistic in vivo with immune checkpoint inhibitors (standard-of-care immuno-therapy agents in oncology) THIO was active in cancer cells that are resistant to tyrosine kinase inhibitors (TKIs), checkpoint inhibitors, IL-2, IFNα, YERVOY® (ipilimumab) and chemotherapy THIO has shown eradication of tumor when administered in sequential combination with checkpoint inhibitors in in vivo syngeneic tumor models of lung, colorectal, and liver cancers THIO has been evaluated and shown high complete response rates and no recurrence in multiple tumor types: IN VITRO: IN VIVO: Lung Colorectal Prostate Breast Ovarian Head and Neck Brain Melanoma Liver Neuroblastoma Pancreatic Brain Lung Liver Melanoma Neuroblastoma Colorecta *Detailed data sets for in vivo/in vivo studies are available for further reviews MAIA BIOTECHNOLOGY