
Issuer Free Writing Prospectus
Filed Pursuant to Rule 433
Registration Statement No. 333-269606
Dated April 20, 2023
(To Preliminary Prospectus dated April 19, 2023)
Free Writing Prospectus
MAIA Biotechnology, Inc.
This free writing prospectus relates to the proposed public offering of shares of common stock, par value $0.0001 of MAIA Biotechnology, Inc. (the “Company”), which are being registered on a Registration Statement on Form S-1, as amended (No. 333-269606) (the “Registration Statement”). This free writing prospectus should be read together with the preliminary prospectus dated April 19, 2023 included in that Registration Statement, which can be accessed through the following link:
https://www.sec.gov/Archives/edgar/data/1878313/000156459023005968/maia-s1a.htm
We have filed the Registration Statement with the Securities and Exchange Commission (the “SEC”) for the offering to which this communication relates. Before you invest, you should read the preliminary prospectus in the Registration Statement (including the risk factors described therein) and other documents we have filed with the SEC for more complete information about our Company and this offering. You may access these documents for free by visiting EDGAR on the SEC Web site at http://www.sec.gov. Alternatively, we or any underwriter participating in the offering will arrange to send you the prospectus if you contact ThinkEquity, Prospectus Department, 17 State Street, 41st Floor, New York, New York 10004, telephone: (877) 436-3673 or e-mail: prospectus@think-equity.com.

MAIA BIOTECHNOLOGY TELOMERE TARGETING IMMUNOTHERAPIES FOR CANCER NYSE AMERICAN: MAIA April 2023

FREE WRITING PROSPECTUS This presentation highlights basic information about us and the proposed offering. Because it is a summary, it does not contain all of the information that you should consider before investing. We have filed a registration statement (including a prospectus) with the SEC for the offering to which this presentation relates. The registration statement has not yet become effective. Before you invest, you should read the prospectus in the registration statement (including the risk factors described therein) and other documents we have filed with the SEC for more complete information about us and the offering. You may access these documents for free by visiting EDGAR on the SEC Web site at http://www.sec.gov. Alternatively, we or any underwriter participating in the offering will arrange to send you the prospectus if you contact ThinkEquity, Prospectus Department, 17 State Street, 41st Floor, New York, New York 10004, telephone: (877) 436-3673 or e-mail: prospectus@think- equity.com. This presentation shall not constitute an offer to sell, or the solicitation of an offer to buy, nor will there be any sale of these securities in any state or other jurisdiction in which such offer, solicitation or sale would be unlawful prior to the registration or qualification under the securities laws of such state or jurisdiction. The offering will only be made by means of a prospectus pursuant to a registration statement that is filed with the SEC after such registration statement becomes effective. 2

FORWARD LOOKING STATEMENTS All statements in this presentation, other than those relating to historical facts, are "forward-looking statements." These forward-looking statements may include, but are not limited to, statements relating to our objectives, plans, and strategies; statements that contain projections of results of operations or of financial condition; statements relating to the industry and government policies and regulations relating to our industry; and all statements (other than statements of historical facts) that address activities, events, or developments that we intend, expect, project, believe, or anticipate will or may occur in the future. Forward-looking statements are not guarantees of future performance and are subject to risks and uncertainties. We have based these forward-looking statements on assumptions and assessments made by our management in light of their experience and their perception of historical trends, current conditions, expected future developments, and other factors they believe to be appropriate. Important factors that could cause actual results, developments, and business decisions to differ materially from those anticipated in these forward-looking statements include, among other things: the overall global economic environment; general market, political, and economic conditions in the countries in which we operate: projected capital expenditures and liquidity; changes in our strategy; government regulations and approvals; the application of certain service license; and litigation and regulatory proceedings. The Company has filed a registration statement on Form S-1, as may be amended (Registration No.: 333-269606). Before you invest, you should carefully read the registration statement, including the factors described in the “RISK FACTORS” section of the Registration Statement and other documents that we have filed, and will subsequently file, with the Securities and Exchange Commission to better understand the risks and uncertainties inherent in our business and industry and for more complete information about us and the offering. You may get these documents for free by visiting EDGAR on the Commission's website at www.sec.gov. We caution you that forward-looking statements are not guarantees of future performance and that our actual results of operations, financial condition and liquidity, and the development of the industry in which we operate may differ materially from the forward-looking statements contained in this presentation as a result of, among other factors, the factors referenced in the "Risk Factors” section of the Registration Statement. In addition, even if our results of operations, financial condition and liquidity, and the development of the industry in which we operate are consistent with the forward-looking statements contained in this presentation, they may not be predictive of results or developments in future periods. This presentation shall not constitute an offer to sell or the solicitation of an offer to sell or the solicitation of an offer to buy any of our securities nor shall there be any sale of securities in any jurisdiction in which such offer, solicitation or sale would be unlawful prior to registration or qualification under the securities laws of any such jurisdiction. Any offering of securities can only be made in compliance with applicable securities laws. You should read carefully the factors described in the “Risk Factors" section of the Registration Statement to better understand the risks and uncertainties inherent in our business and underlying any forward-looking statements. These statements are only current predictions and are subject to known and unknown risks, uncertainties, and other factors that may cause our or our industry's actual results, levels of activity, performance, or achievements to be materially different from those anticipated by the forward-looking statements. You should not rely upon forward-looking statements as predictions of future events. Although we believe that the expectations reflected in the forward-looking statements are reasonable, we cannot guarantee future results, levels of activity, performance, or achievements. Except as required by law, we are under no duty to update or revise any of the forward-looking statements, whether as a result of new information, future events or otherwise, after the date of this prospectus. These forward-looking statements speak only as of the date of this presentation, and we assume no obligation to update or revise these forward-looking statements for any reason. 3

INVESTMENT OVERVIEW • Telomere-Targeting Agents: o THIO in clinic o Advancing pipeline • Efficacy • Safety • FDA: 2 Orphan Drug Designations • REGN: Clinical Supply Agreement • Phase 2 THIO-101 trial in NSCLC underway o Enrolling in AUS and EU o On track to open sites in US in 2023 o Upcoming Milestones: Safety, ORR, DoR • Phase 2 THIO-102 basket/umbrella trial in 2023 • THIO-103 basket trial in 2023 4
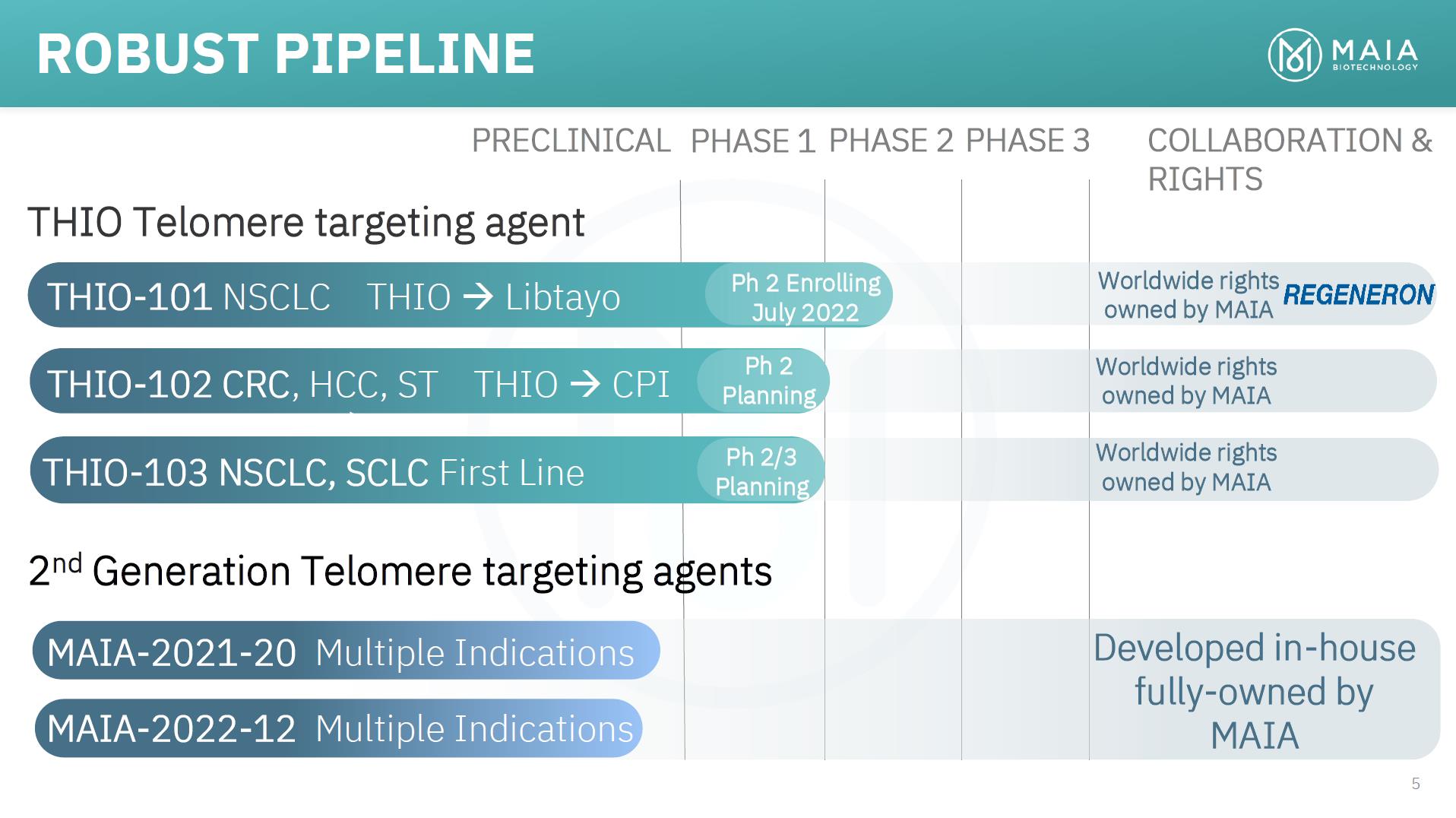
ROBUST PIPELINE PRECLINICAL PHASE 1 PHASE 2 PHASE 3 COLLABORATION & RIGHTS THIO Telomere targeting agent THIO-101 NSCLC THIO → Libtayo THIO-102 CRC, HCC, ST THIO → CPI THIO-103 NSCLC, SCLC First Line Ph 2 Enrolling July 2022 Ph 2 Planning Ph 2/3 Planning Worldwide rights owned by MAIA Worldwide rights owned by MAIA Worldwide rights owned by MAIA 2nd Generation Telomere targeting agents MAIA-2021-20 Multiple Indications MAIA-2022-12 Multiple Indications Developed in-house fully-owned by MAIA 5

SCIENCE OVERVIEW THIO (6-thio-dG) Telomere Targeting Agent • Small molecule (penetrates blood-brain barrier) • Eligible for NCE marketing exclusivity • Dual MoA: telomere targeting + immunogenic • Complete Response with No Recurrence in vivo in Lung, Colorectal, Liver, Melanoma, Brain Cancer (GBM, DIPG, MB), etc • FDA Orphan Drug Designations: HCC and SCLC Next Generation Telomere Targeting Candidates • Similar MoA • Structures: evolution of THIO; other new structures • Objective: advance to pre-IND development one agent every 12 months 6

MISSION AND APPROACH 7
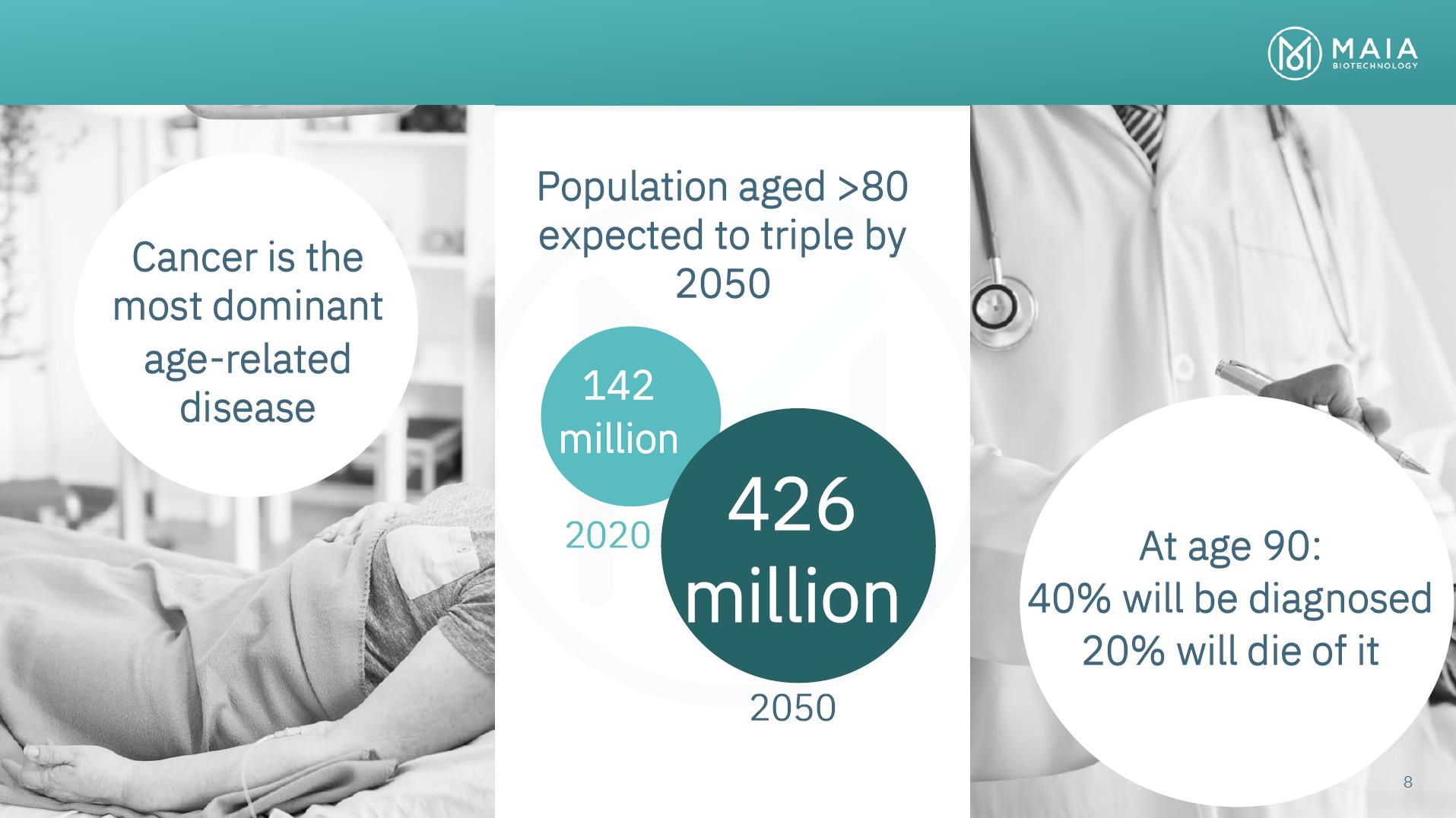
Cancer is the most dominant age-related disease Population aged >80 expected to triple by 2050 142 million 2020 426 million 2050 At age 90: 40% will be diagnosed 20% will die of it 8

THIO is the only direct telomere targeting agent currently in clinical development 9
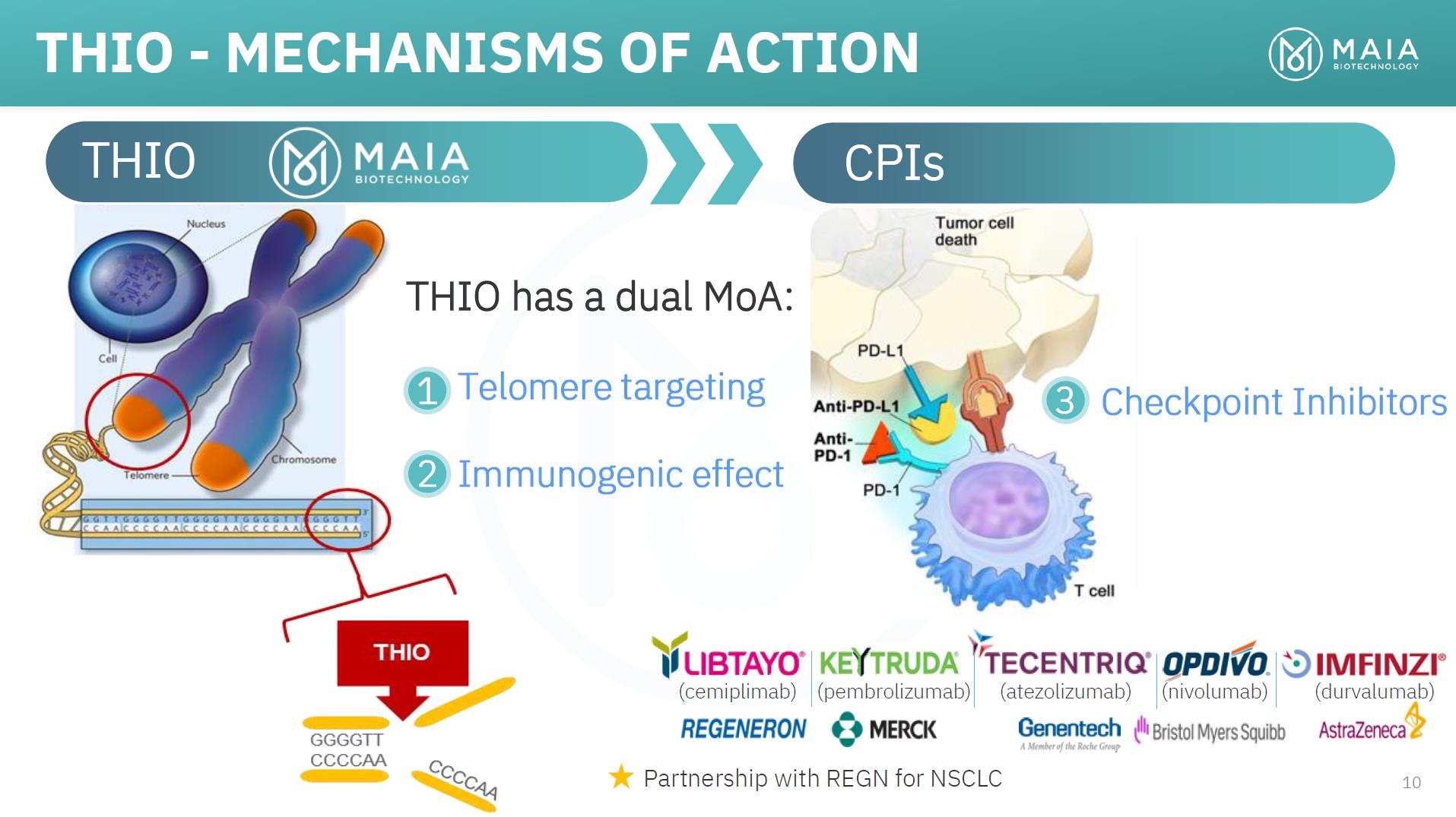
THIO - MECHANISMS OF ACTION THIO CPIs THIO has a dual MoA: 1 Telomere targeting 2 Immunogenic effect 3 Checkpoint Inhibitors (cemiplimab) (pembrolizumab) (atezolizumab) (nivolumab) (durvalumab) Partnership with REGN for NSCLC 10

MAIA BIOTECHNOLOGY & REGENERON MAIA Biotechnology, Inc. Announces Clinical Supply Agreement with Regeneron for Phase 1/2 Clinical Trial Evaluating THIO in Sequential Administration with Libtayo(R) (cemplimab) in Advanced Non-Small Cell Lung Cancer 11
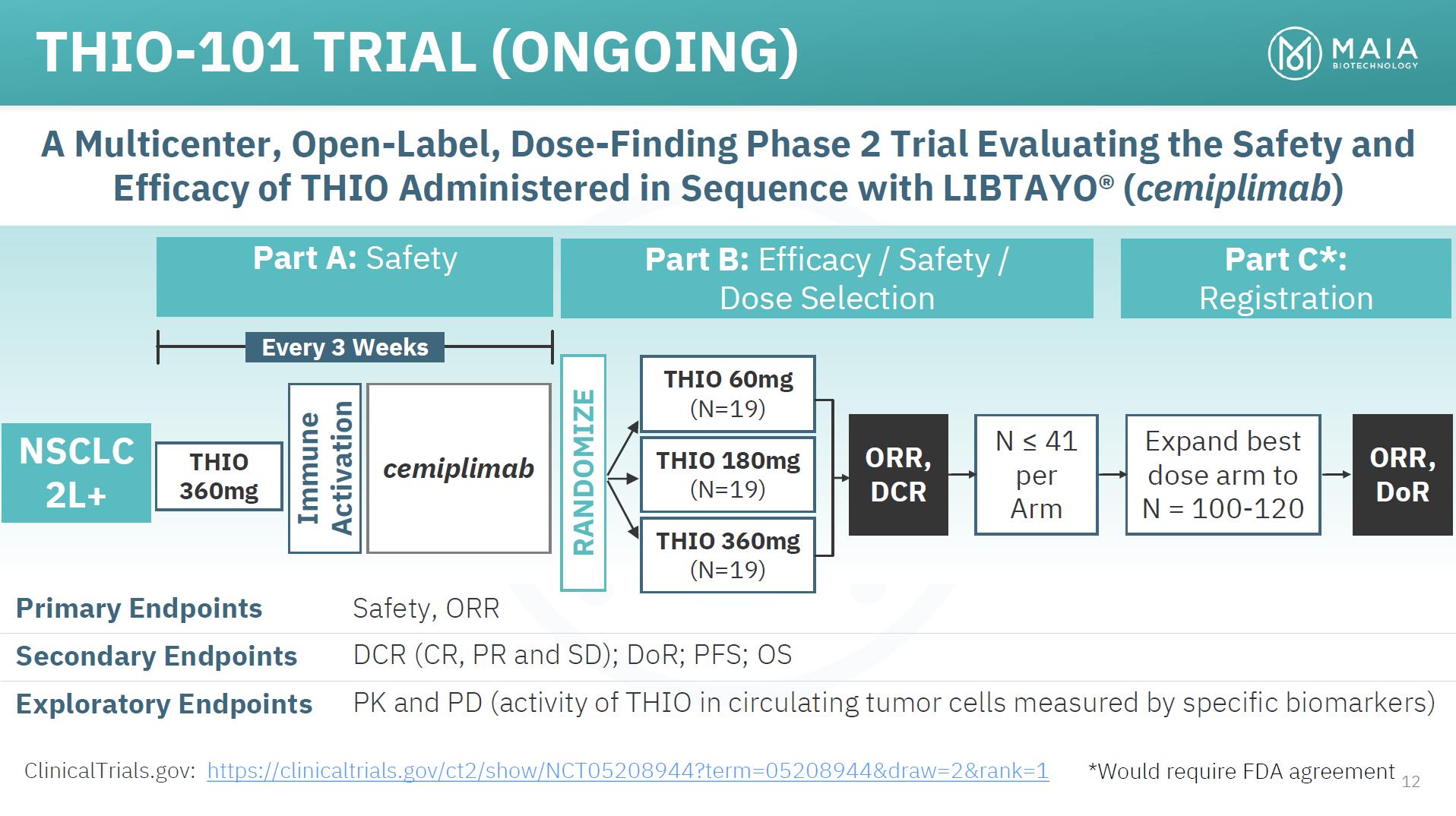
THIO-101 TRIAL (ONGOING) A Multicenter, Open-Label, Dose-Finding Phase 2 Trial Evaluating the Safety and Efficacy of THIO Administered in Sequence with LIBTAYO® (cemiplimab) Part A: Safety Part B: Efficacy / Safety / Dose Selection Part C*: Registration Every 3 Weeks NSCLC 2L+ THIO 360mg cemiplimab ORR, DCR N ≤ 41 per Arm Expand best dose arm to N = 100-120 ORR, DoR Primary Endpoints Safety, ORR Secondary Endpoints DCR (CR, PR and SD); DoR; PFS; OS Exploratory Endpoints PK and PD (activity of THIO in circulating tumor cells measured by specific biomarkers) ClinicalTrials.gov: https://clinicaltrials.gov/ct2/show/NCT05208944?term=05208944&draw=2&rank=1 *Would require FDA agreement 12

FAVORABLE SAFETY PROFILE • Safety events reported during dose-limiting toxicity window • 360 mg/cycle – THIO highest dose • Data from 6 patients who completed the dose-limiting toxicity (DLT) period in Cycle 1 (3 weeks) • No Serious Adverse Events (SAE) or Serious Unexpected Suspected Adverse Reactions (SUSAR) • Safety profile substantially better than current standard of care • Chemotherapy has 70-80% incidence of grade 3-4 very severe side effects • Started Part B (efficacy/dose selection) of the trial upon recommendation by the Safety Review Committee 13

FAVORABLE SAFETY PROFILE Adverse events (AE) reported – DLT window (3 weeks) Grade Fatigue 1 Decreased appetite 1 Blood pressure fluctuation 1 Dyspnea 1 Nausea 1 Interleukin-6 (IL-6) level increased* 1 Rash erythematous 1 Constipation 1 Myalgia 1 Vomiting 2 Nausea** 3 *T-cell activation **Resolved within 72 hours with treatment 14

PRELIMINARY SURVIVAL DATA • The first 2 patients enrolled in Part A of the study continue to be alive, approximately 10 and 9 months respectively, from treatment initiation • Both patients have advanced Stage IV metastatic disease and are heavily pretreated, receiving third and fourth line of therapy respectively after previously failing treatment with an immune checkpoint inhibitor • They continue to be progression free following their last dose, 7 and 6 months respectively, with no new treatment • In real-world clinical practice, observed survival in such heavily pretreated patients is 3-4 months 15
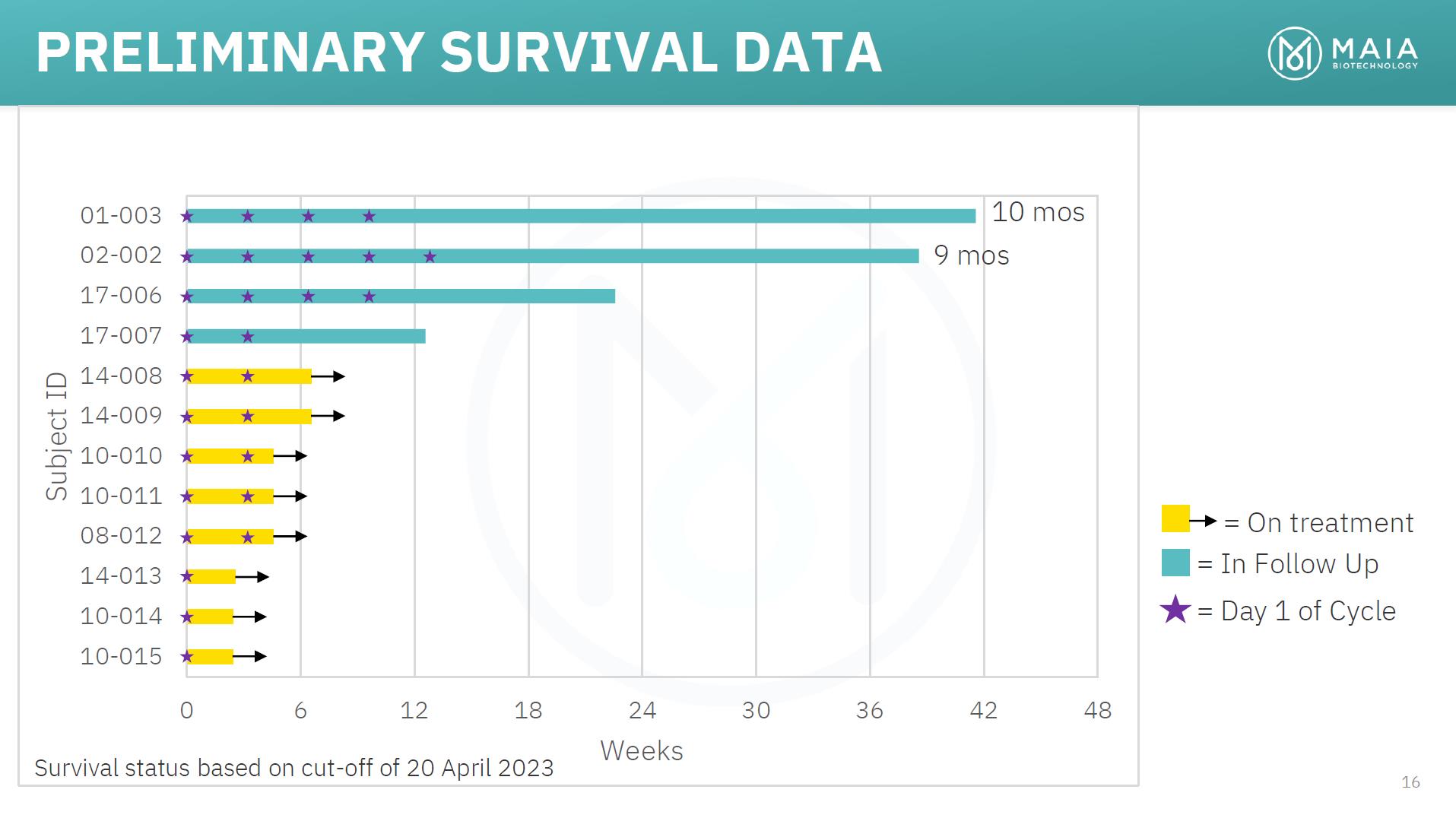
PRELIMINARY SURVIVAL DATA 01-003 02-002 17-006 17-007 14-008 14-009 10-010 10-011 08-012 14-013 10-014 10-015 10 mos 9 mos = On treatment = In Follow Up = Day 1 of Cycle 0 6 12 18 24 30 36 42 48 Weeks Survival status based on cut-off of 20 April 2023 16

THIO followed by CPI results in 60% complete response • No recurrence after long-term follow-up • Anticancer immune memory has been induced: no cancer after rechallenge with 5x more lung cancer (LLC) cells with no additional therapy 1000 900 800 700 600 500 400 300 200 100 0 Control Atezolizumab THIO THIO Atezo No cancer immune memory Rechallenge with due to THIO 5x more lung cancer cells with no additional treatment No recurrence 20% PR 60% CR 0 10 20 30 40 50 60 70 80 90 Days After Tumor Inoculation Mender et al, Cancer Cell, 2020; THIO followed by Tecentriq (atezolizumab; Roche/Genentech) tested first; repeated later with THIO followed by Keytruda (pembrolizumab; Merck); and Libtayo (cemiplimab; Regeneron) 17
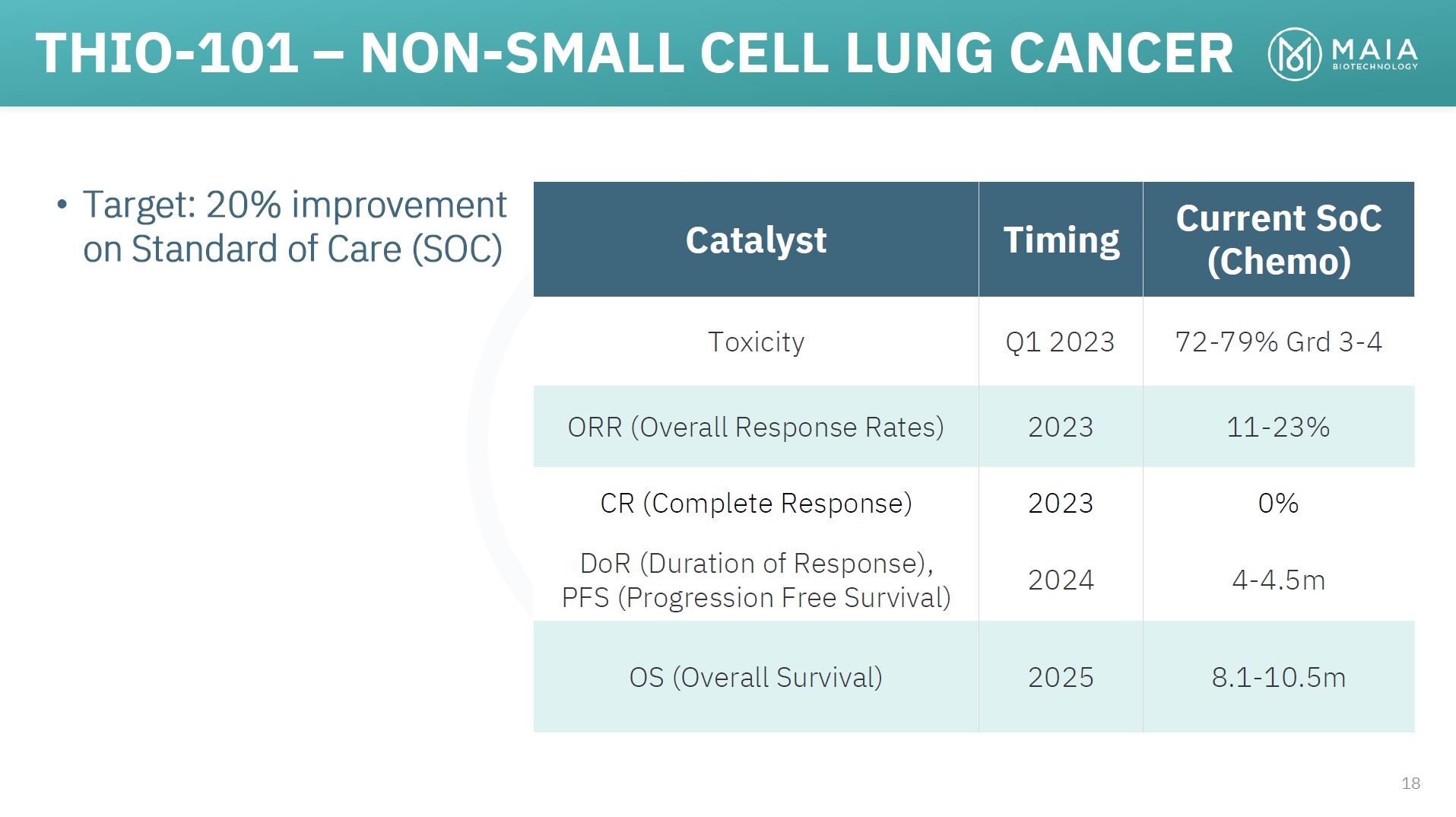
THIO-101 – NON-SMALL CELL LUNG CANCER • Target: 20% improvement on Standard of Care (SOC) Catalyst Timing Current SoC (Chemo) Toxicity Q1 2023 72-79% Grd 3-4 ORR (Overall Response Rates) 2023 11-23% CR (Complete Response) 2023 0% DoR (Duration of Response), PFS (Progression Free Survival) 2024 4-4.5m OS (Overall Survival) 2025 8.1-10.5m 18

BIOMARKER – TIFS (TELOMERE DYSFUNCTION INDUCED FOCI) Confocal microscopy image of LLC cell nucleus after treatment with THIO Quantification of TIFs induced in LLC cell by 3 µM of THIO DAPI γH2AX Tel C • Yellow dots indicated TIFs by THIO • Green dots - γH2AX • Red dots - telomeres THIO • TIFs induction reached max after ~ 48h • Formation of TIFs indicated on-target MOA of THIO 19
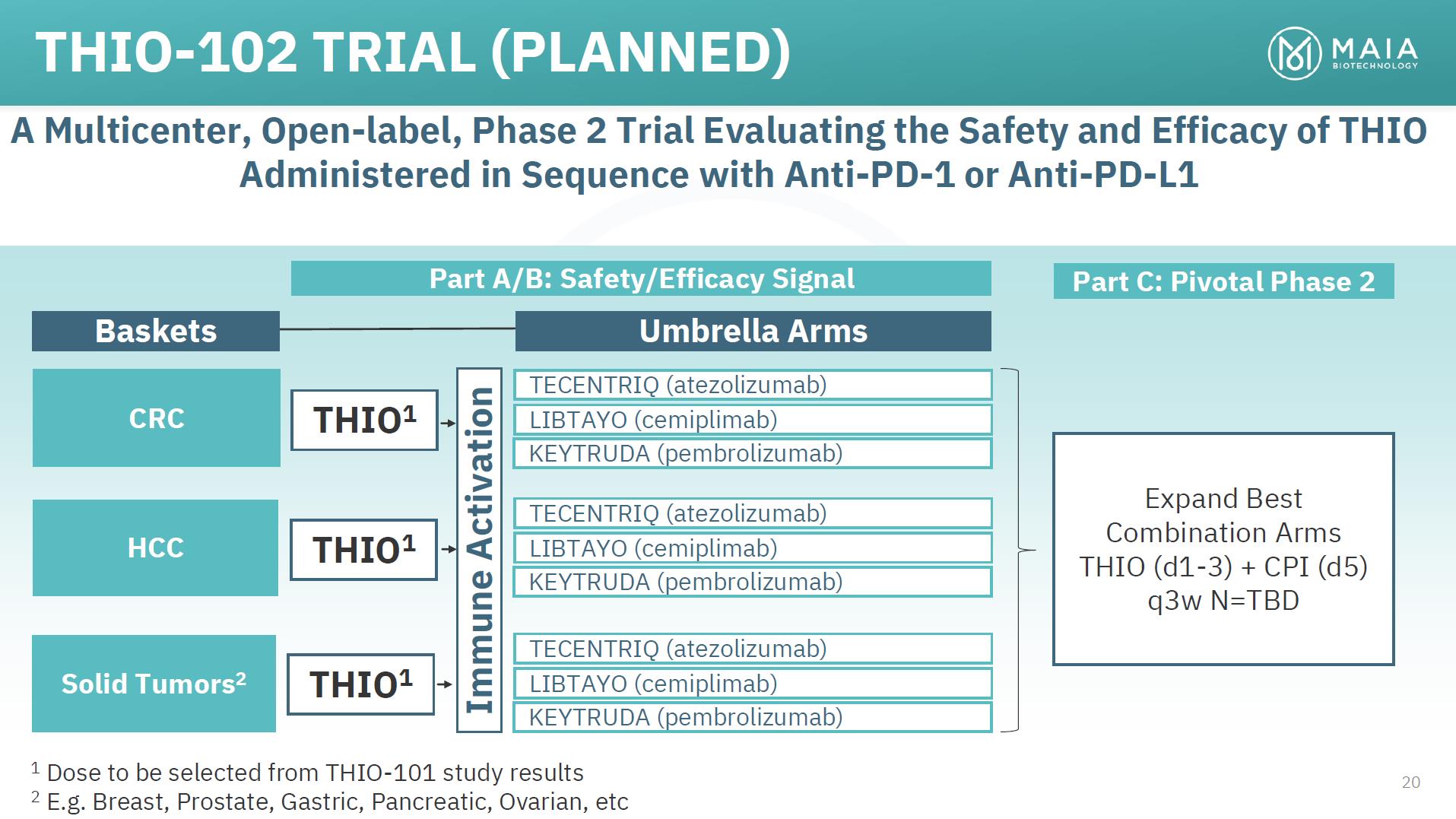
THIO-102 TRIAL (PLANNED) A Multicenter, Open-label, Phase 2 Trial Evaluating the Safety and Efficacy of THIO Administered in Sequence with Anti-PD-1 or Anti-PD-L1 Part A/B: Safety/Efficacy Signal Part C: Pivotal Phase 2 Baskets Umbrella Arms CRC HCC Solid Tumors2 THIO1 THIO1 THIO1 TECENTRIQ (atezolizumab) LIBTAYO (cemiplimab) KEYTRUDA (pembrolizumab) TECENTRIQ (atezolizumab) LIBTAYO (cemiplimab) KEYTRUDA (pembrolizumab) TECENTRIQ (atezolizumab) LIBTAYO (cemiplimab) KEYTRUDA (pembrolizumab) Expand Best Combination Arms THIO (d1-3) + CPI (d5) q3w N=TBD 1 Dose to be selected from THIO-101 study results 20 2 E.g. Breast, Prostate, Gastric, Pancreatic, Ovarian, etc
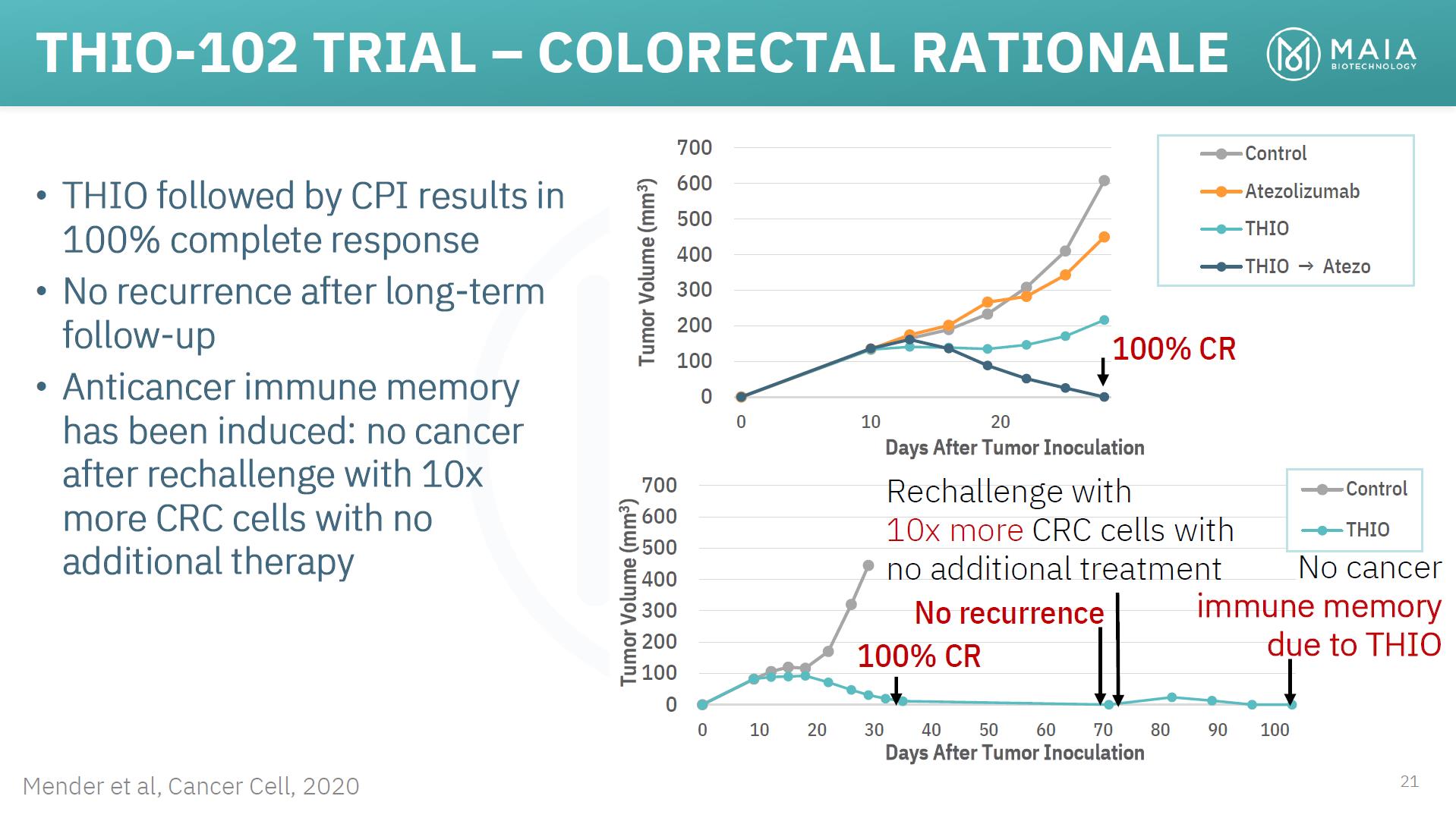
THIO-102 TRIAL – COLORECTAL RATIONALE • THIO followed by CPI results in 100% complete response • No recurrence after long-term follow-up • Anticancer immune memory has been induced: no cancer 700 600 500 400 300 200 100 0 0 10 20 100% CR Control Atezolizumab THIO THIO → Atezo after rechallenge with 10x more CRC cells with no additional therapy 700 600 500 400 Days After Tumor Inoculation Rechallenge with 10x more CRC cells with no additional treatment Control THIO No cancer 300 200 100 0 No recurrence 100% CR immune memory due to THIO Mender et al, Cancer Cell, 2020 0 10 20 30 40 50 60 70 80 90 100 Days After Tumor Inoculation 21

THIO-102 TRIAL - COLORECTAL • Target: 20% improvement on Standard of Care (SOC) Catalyst Timing Current SoC (Chemo) Toxicity 2024 50-60% Grd ≥ 3 ORR 2024 1-1.6% DoR, PFS 2025 1.9-2.0m OS 2026 6.4-7.2m 22

SCLC & HCC – ORPHAN DRUG DESIGNATION 1500 1250 1000 750 500 250 0 SCLC 0 5 10 15 20 25 Days After Tumor Inoculation Control Pembro → THIO THIO → Pembro 1500 1250 1000 750 500 250 0 HCC 0 5 10 15 20 25 Days After Tumor Inoculation Control THIO THIO → IR IR + Atezo THIO → IR + Atezo • THIO is synergistic with anti-PD-1 agent Pembrolizumab in Small Cell Lung Carcinoma (H2081) in vivo in humanized murine cancer model. • Treatment with THIO followed by Pembrolizumab results in highly potent anticancer effect, as compared to Pembrolizumab alone. • THIO converts immunologically “cold non-responsive” SCLC tumor into “hot and responsive” to Pembrolizumab. • THIO is highly synergistic and effective in combination with anti-PD-L1 agent Atezolizumab and Ionizing Radiation (IR 10Gy) in HCC53N Hepatocellular Carcinoma. • Treatment with THIO in combination with IR and Atezolizumab results in a complete regression of aggressive HCC tumors. The combination of IR and Atezolizumab is just partially efficacious. 23
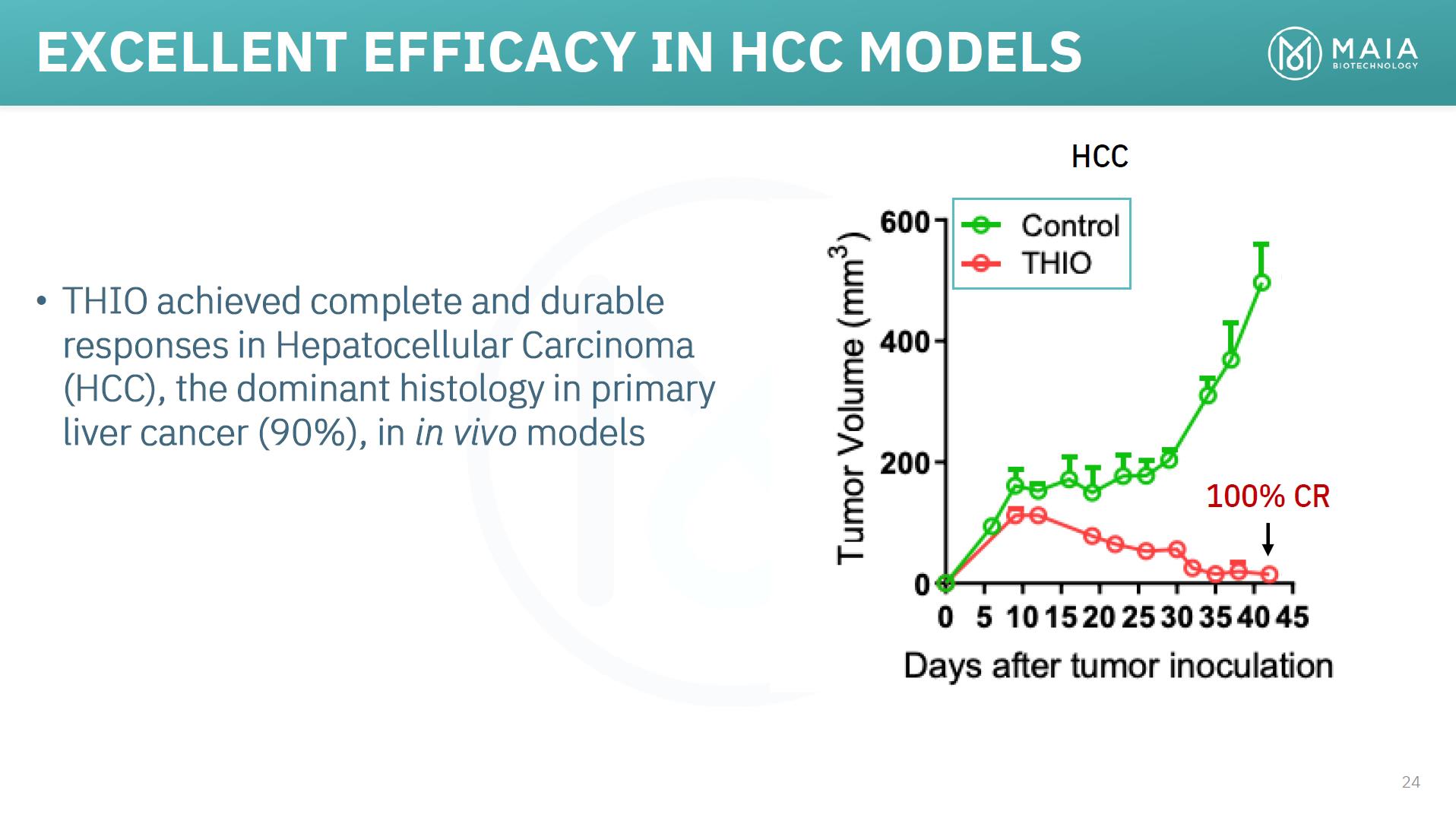
EXCELLENT EFFICACY IN HCC MODELS HCC • THIO achieved complete and durable responses in Hepatocellular Carcinoma (HCC), the dominant histology in primary liver cancer (90%), in in vivo models 100% CR 24

HCC ANTI-CANCER IMMUNE MEMORY • When combined with immunotherapy checkpoint inhibitor Libtayo®, duration of response was further potentiated HCC • Upon rechallenge with two times more cancer cells and no additional treatment, tumor growth was completely prevented Rechallenge with HCC • Administration of THIO alone and in combination with Libtayo® generated anti-cancer immune memory 2x more HCC cells with no additional treatment No cancer immune memory due to THIO 25
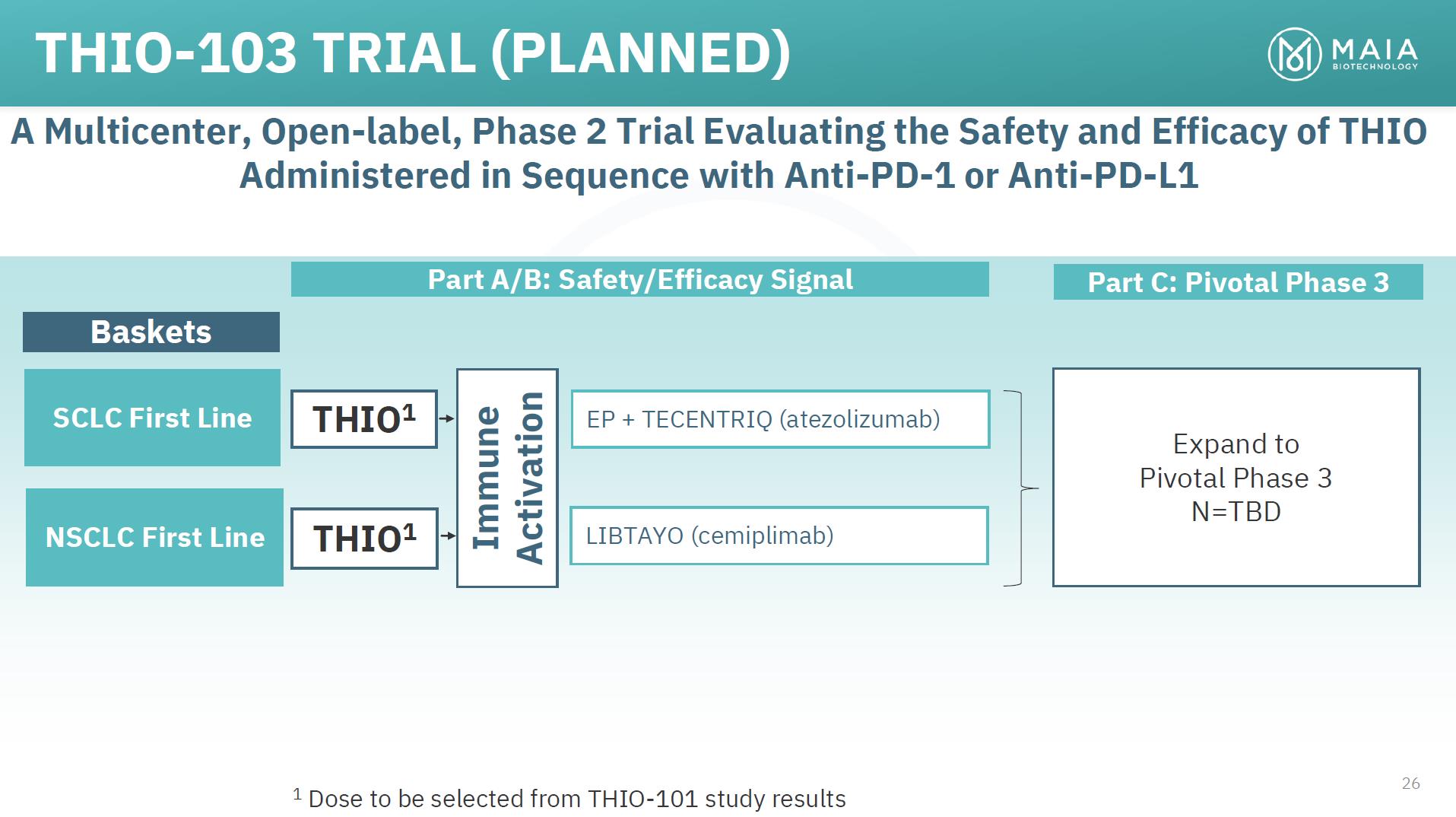
THIO-103 TRIAL (PLANNED) A Multicenter, Open-label, Phase 2 Trial Evaluating the Safety and Efficacy of THIO Administered in Sequence with Anti-PD-1 or Anti-PD-L1 Baskets Part A/B: Safety/Efficacy Signal Part C: Pivotal Phase 3 SCLC First Line NSCLC First Line THIO1 THIO1 EP + TECENTRIQ (atezolizumab) LIBTAYO (cemiplimab) Expand to Pivotal Phase 3 N=TBD 26 1 Dose to be selected from THIO-101 study results

EXCLUSIVITY AND INTELLECTUAL PROPERTY Goal: New Chemical Entity (NCE) Marketing Exclusivity • THIO has never been previously approved by the FDA for commercialization • Robust exclusivity • US: 7 years; EU, Japan, other markets: 10 years Robust and Growing Patent Portfolio for THIO • 1 issued US patent • 4 issued foreign patents • 5 pending US patent applications • 7 pending foreign patent applications Current patents/provisional applications broadly cover the following key areas: • Telomere targeting compounds (2034+) • THIO’s immunogenic treatment strategy: sequential combination with CPIs (2041) 27

EXPERIENCED MANAGEMENT TEAM Vlad Vitoc, MD, MBA Founder, Chairman, and Chief Executive Officer • 22+ years in Oncology Pharma/ Biotech: Commercial, Medical • 12 compounds launched across 20+ tumor types • Leadership roles at Bayer (Nexavar), Astellas (Tarceva, Xtandi), Cephalon (Treanda), Novartis (Zometa), and Incyte (Jakafi) Mihail Obrocea, MD Chief Medical Officer • Hematologist/Oncologist executive • 21+ years of drug development experience: cell therapy, active immunotherapy and cancer vaccines, antibodies, antibody drug conjugates (ADCs), small molecules Sergei Gryaznov, PhD Chief Scientific Officer • 25+ years as Scientist • Expert Drug Discovery and Development, Oncology with 120+ publications • Head of the J&J Oligonucleotide Center of Excellence Worldwide • Expert of telomeres and telomerase in cancer, co- inventor of THIO Joe McGuire Chief Financial Officer • 30+ years of financial expertise • CFO for privately held and publicly traded companies in the healthcare and other industries 28
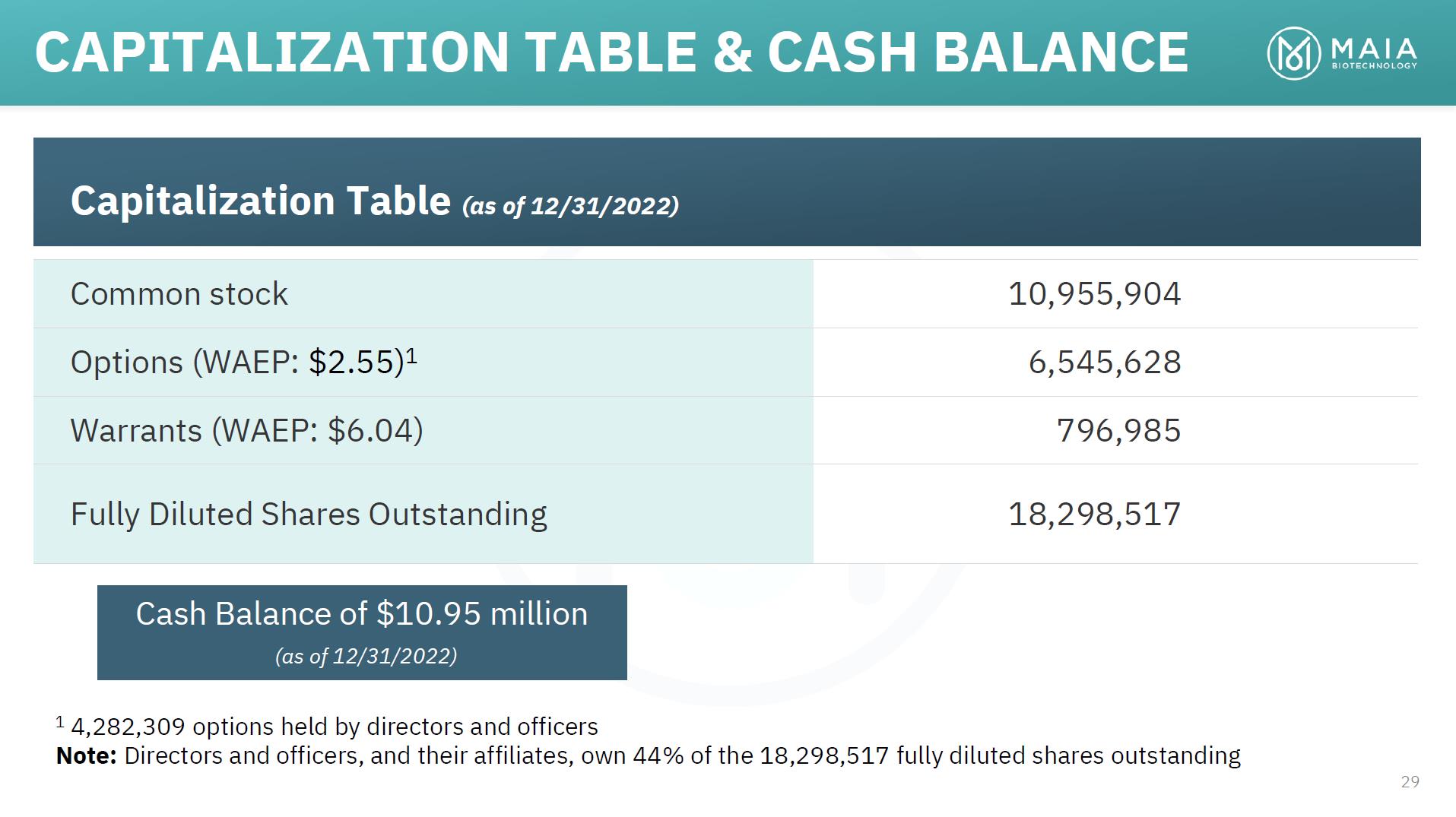
Capitalization Table (as of 12/31/2022) Common stock 10,955,904 Options (WAEP: $2.55)1 6,545,628 Warrants (WAEP: $6.04) 796,985 Fully Diluted Shares Outstanding 18,298,517 Cash Balance of $10.95 million (as of 12/31/2022) 1 4,282,309 options held by directors and officers Note: Directors and officers, and their affiliates, own 44% of the 18,298,517 fully diluted shares outstanding 29

INVESTMENT OPPORTUNITY 30

SIGNIFICANT MARKET OPPORTUNITY Developing agents for the top tumor types markets globally $42B… NSCLC #1 WW Mortality: 1.7M Sales: $ 34B CRC #2 WW Mortality: 1.0M Sales: $ 20B $42B CPIs Group • 5 CPIs approved for NSCLC sold $12B • >30% of NSCLC drug sales • >40% of total CPI sales • Keytruda®: $7.5B in NSCLC of $21B total Checkpoint Inhibitors $0.5B Partnership with Regeneron (Libtayo®) • Profile similar to Keytruda® • Libtayo® is entrant #5 in CPIs • Needs superior efficacy to Keytruda® Keytruda® (pembrolizumab) Opdivo® (nivolumab) Tecentriq® (atezolizumab) Imfinzi® (durvalumab) Libtayo® (cemiplimab) • Sequential combination with THIO is key 31
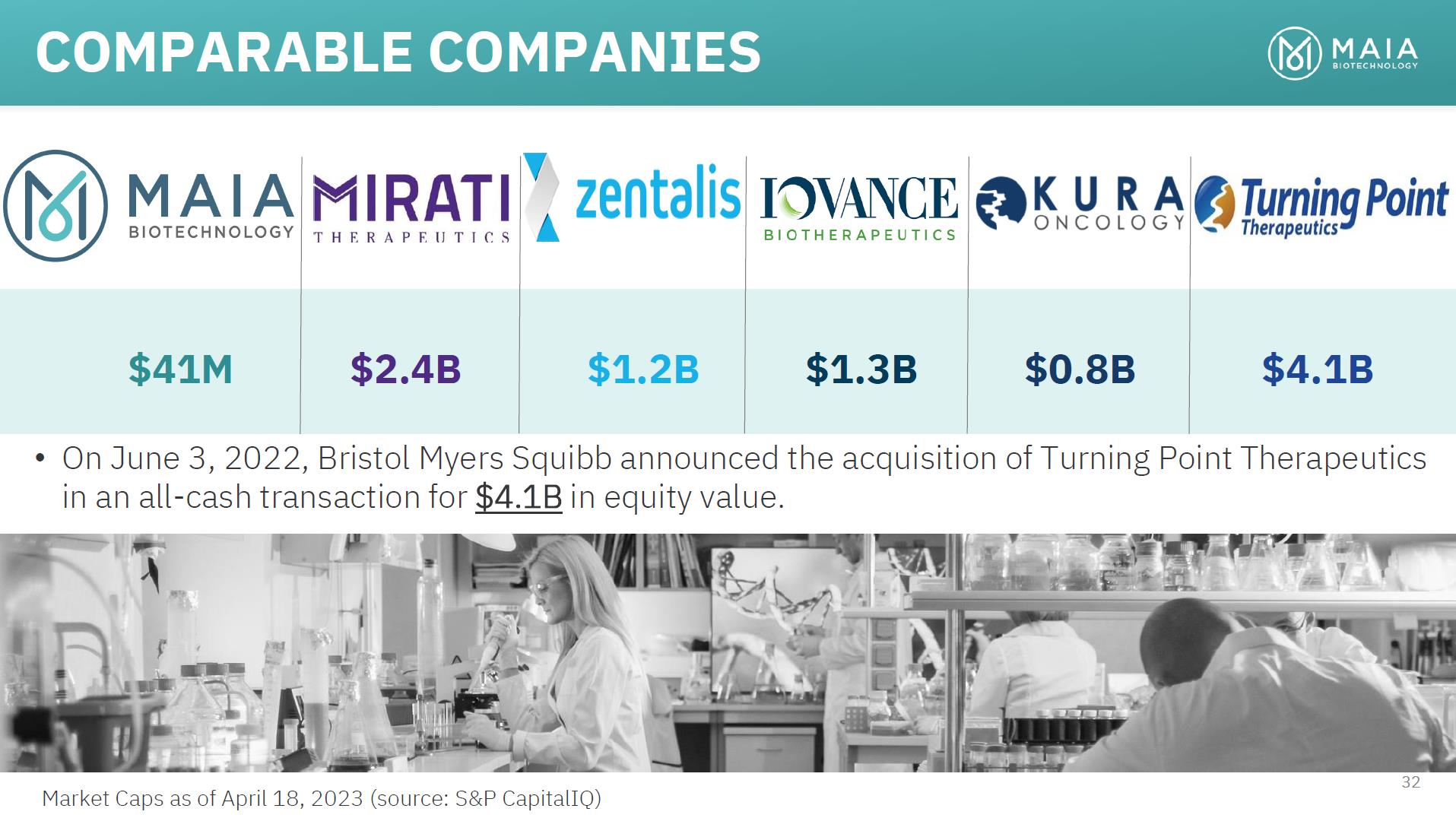
COMPARABLE COMPANIES $41M $2.4B $1.2B $1.3B $0.8B $4.1B • On June 3, 2022, Bristol Myers Squibb announced the acquisition of Turning Point Therapeutics in an all-cash transaction for $4.1B in equity value. 32 Market Caps as of April 18, 2023 (source: S&P CapitalIQ)
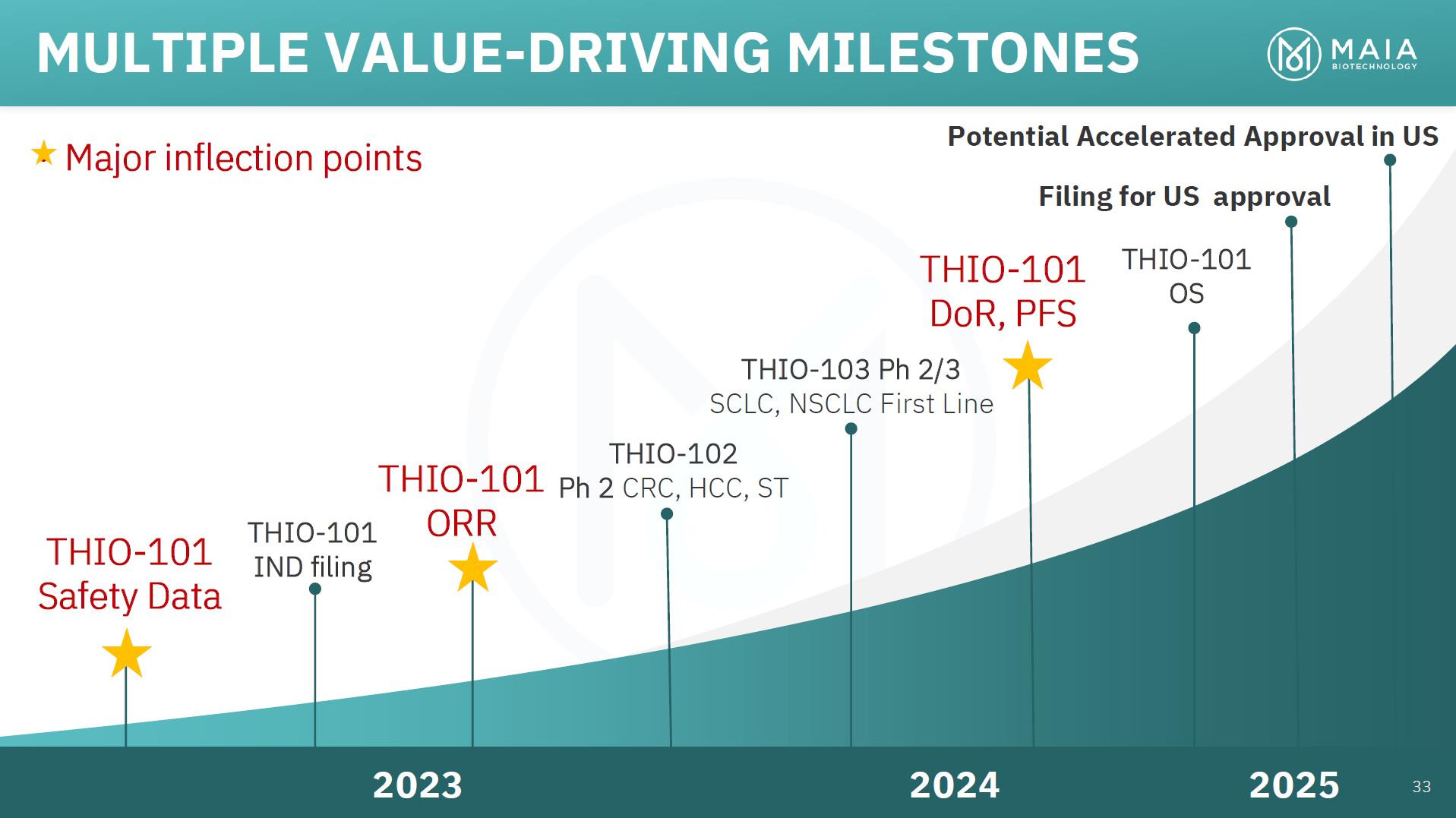
MULTIPLE VALUE-DRIVING MILESTONES • Major inflection points Potential Accelerated Approval in US Filing for US approval THIO-101 Safety Data THIO-101 IND filing THIO-101 ORR THIO-101 DoR, PFS THIO-103 Ph 2/3 SCLC, NSCLC First Line THIO-102 Ph 2 CRC, HCC, ST THIO-101 OS 2023 2024 2025 33

NYSE: MAIA July 28, 2022 34